MetP Pharma has announced new preclinical data demonstrating that its proprietary nasal technology achieves rapid, dose-dependent brain targeting of GLP-1-based drugs (such as semaglutide) while maintaining low systemic exposure.
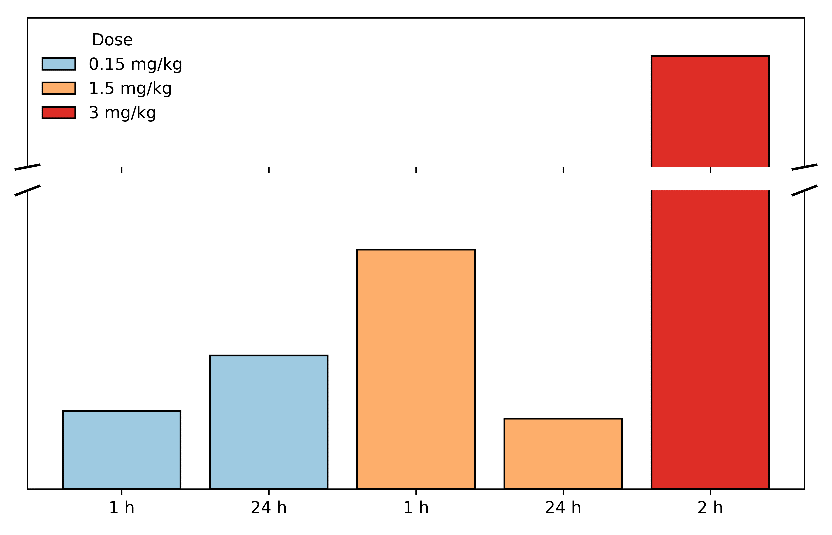
In a 2025 preclinical study in rats, intranasal administration of semaglutide using MetP Pharma’s technology resulted in brain-to-plasma ratios consistently greater than 1, confirming preferential delivery to the central nervous system (CNS).
Brain exposure increased proportionally with dose and was observed rapidly after administration, highlighting the robustness and controllability of the platform.
A brain-to-plasma ratio > 1 for semaglutide has never been described with subcutaneous/oral/nasal administration and is extremely unusual — according to current knowledge, it is almost impossible.
In contrast, preclinical studies in rats following subcutaneous or oral semaglutide administration demonstrate only a very limited brain penetration.
A brain-to-plasma ratio > 1 with MetP Pharma’s nasal technology suggests that semaglutide is actively accumulated in the brain, leading to strong central satiety and appetite suppression.
"These data reinforce our belief that effective CNS targeting is achievable without high systemic exposure," said Dr Claudia Mattern, Chief Scientific Officer of MetP Pharma.
"By shifting GLP-1 exposure toward the brain and away from the periphery, our technology directly addresses key limitations of current injectable and oral GLP-1 therapies."
A next-generation GLP-1 strategy
Current subcutaneous and oral GLP-1 receptor agonists generate high plasma levels but achieve only limited direct brain exposure, contributing to dose-limiting gastrointestinal side effects and restricting their broader use in CNS-driven indications.
MetP Pharma’s nasal enabling technology is non-invasive, designed for easy self-administration and leverages direct nose-to-brain transport pathways that bypass the blood–brain barrier to overcome these limitations.
The platform enables several strategic opportunities, including the following:
- Lifecycle extension and differentiation
- Improved benefit-risk profile
- Scalable platform across CNS indications
Building on a validated nose-to-brain platform
The new in vivo study results build on MetP Pharma’s extensive preclinical body of evidence demonstrating rapid and sustained CNS exposure of GLP-1–based therapeutics using its proprietary nasal technologies.
Together, these results position MetP Pharma’s platform as a versatile enabling solution for the next generation of CNS-active metabolic drugs.
"GLP-1 biology is fundamentally linked to the brain," Dr Mattern added.
"Our approach is designed to unlock that potential more directly, opening new therapeutic and commercial possibilities for well-validated molecules."
MetP Pharma is actively exploring partnerships to advance brain-targeted GLP-1 programmes into further preclinical and clinical development.
