As well as summarising the risks of potential microbial and fungal contamination to developers and patients, the authors — Stefan Karl, CEO, Mikrobiologisches Prüflabor GmbH, and Olaf Degen, Director Industry Microbiology at Bruker Microbiology & Infection Diagnostics — also discuss the challenges associated with existing analytical methods.
They then introduce the latest state-of-the-art technology that helps analysts and pharma developers to quickly get the answers they need.
As early adopters of this solution — based on matrix assisted laser desorption/ionisation-time of flight mass spectrometry (MALDI-TOF MS) — they share their experiences and outline the benefits that have been achieved.
Many active pharmaceutical ingredients (APIs) and excipients (such as sugar and cellulose) support microbial growth and, therefore, could contaminate the final drug product; this poses a significant threat to product efficacy and patient safety.
Potential contaminants are defined by the World Health Organization (WHO) as contaminating micro-organisms or source material(s) that are unintentionally introduced into the manufacturing process of a biological product.
These can be bacteria (including mycoplasma), fungi, parasites and viruses. Potential sources for such contaminants include raw materials and components, ineffective cleaning and in-process controls, handling or personnel, container system failures and sterilisation issues.
Water, in particular, poses a significant risk of microbial contamination as it is used throughout raw material and drug product manufacturing — and can be a key ingredient itself in many injectable and oral drugs.
According to data from the US Food and Drug Administration (FDA) Enforcement Report Program, there were 1712 product recalls between 2012 and 2023 in the US that were caused by contamination (Figure 1): 40% of them were categorised as microbial contamination (Figure 2).
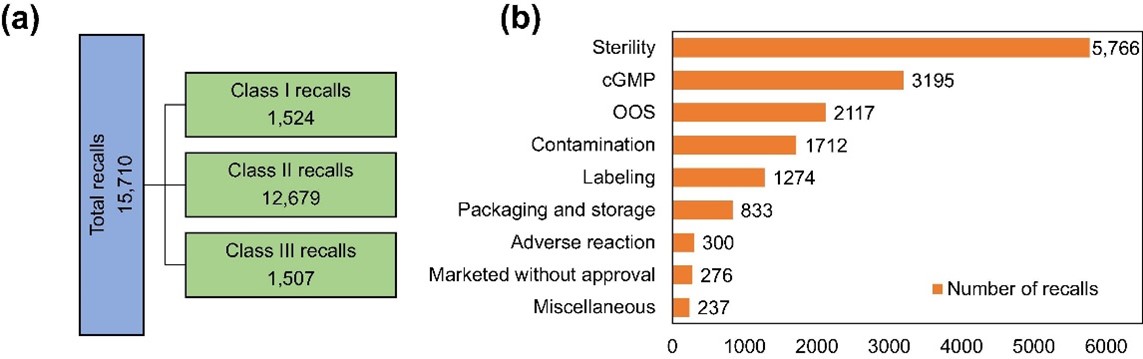
Figure 1: Categorisation of all drug recalls issued by the US FDA from June 2012 to August 2023 (a) and categorisation of drug recalls by key words issued by the USE FDA from 2012 to 2022 (b)3
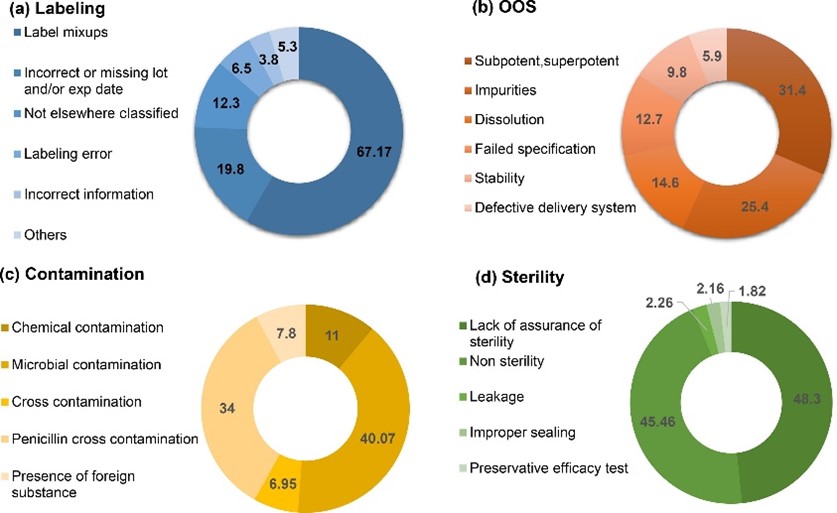
Figure 2: Subcategorisation of recalls caused by labelling (a), OOS (b), contamination (c) and sterility (d) issues3
The presence of microbial contamination in parenteral/sterile dosage forms is classified as a sterility issue, whereas for other formats — such as solutions, suspensions, tablets, ointments and creams — it is listed in the contamination category.
In this highly regulated sector, manufacturers must establish rapid and robust microbial monitoring and contamination control strategies as part of current good manufacturing practice (cGMP).
Owing to the complexity and need for accuracy and speed, product release testing and environmental monitoring are often outsourced to expert laboratories.
Standard techniques take time
Detecting and identifying potential contaminants using genomic techniques, based on polymerase chain reaction (PCR) and deoxyribonucleic acid (DNA) or ribosomal ribonucleic acid (rRNA) sequencing, ensures that raw materials and finished products comply with specification.
Sequencing, however, is time consuming and costly. Additional identification testing typically takes 5–10 hours. Plus, further sample preparation and complex data analysis is required in some instances, which can cause costly delays to product release.
A faster and easier solution is needed that offers the same high level of data quality and integrity.
Outsourced microbial analysis
MPL (Mikrobiologisches Prüflabor) offers validated microbiological analysis and release testing for the pharmaceutical industry, conducted according to the current regulations defined by the European Pharmacopeia (EP) international ISO standards, the United States Pharmacopeia (USP) and by the European Medicines Agency (EMA), all under the cGMP umbrella.
MPL has implemented a new microbial identification technology using the Bruker MALDI Biotyper sirius System that is based on MALDI-TOF MS. The workflow delivers validated results within minutes from a small amount of culture.
The new system at MPL is enhanced by the validated and well-maintained Accugenix reference database of more than 16,000 entries for bacteria and fungi. Comprising Bruker’s library and the Charles River Laboratories in-house library, it ensures reliable and accurate results.
Fungal identification
Fungal contamination can arise from airborne sources or from the workforce. Although less common than bacterial contamination, fungal contamination can have a major impact on the manufacturing process, the product and the patient.
It is coming under increased scrutiny as contaminated pharmaceuticals could lead to outbreaks of severe fungal diseases in the community.1
Identifying moulds and multicellular fungi is one of the most challenging aspects of microbiology and filamentous fungi are notably difficult to detect. In many laboratories worldwide, this process remains fully manual, leading to highly subjective and non-standardised approaches.
The MALDI Biotyper introduces standardisation of analysis, reducing the potential for error compared with even the most diligent manual work and interpretation.
This advancement allows for more efficient testing and reliable cross-referencing with morphological and microscopic assessments.
For next-generation fungal testing, Bruker has introduced the Mycelium Transfer (MyT) procedure with easy direct fungi transfer. After isolating mycelium, the MBT HT Filamentous Fungi Module rapidly identifies challenging filamentous fungi at species level.
The workflow introduces a standardised approach, now using the drying device MBT FAST Shuttle, and combines an extensive reference library with advanced software that supports automated mass spectral acquisition and analysis to deliver accurate identification.
Conclusion
The risk of microbial contamination to pharma developers means that they increasingly rely on expert partners to provide fast and accurate analyses.
If bacterial or fungal isolates are found in a production facility, for example, it is essential to identify the organism rapidly and reliably at species level.
Accurate identification supports tracking, trend reporting and setting up a reliable data log to inform future preventive methods.
Delays in the identification of microbial contamination can hinder production and result in both increased testing requirements and the need for extensive cleaning and validation processes, which increase operational costs significantly.
According to a study led by The London School of Economics (LSE), researchers estimate that between 2009 and 2018 the median cost of bringing a new drug to market was $985 million and the average cost was $1.3 billion.2
The latest technological advances remove the reliance on genomic methods to accelerate microbiological testing, providing rapid/reliable and reproducible results.
This critical link in the pharma supply chain is fundamental to developers as it helps them to reduce manufacturing costs and bring safe, efficacious drugs to the patients who need them.
References
- M.A.E.E. Ahmed, H.S. Abbas and M. Kotakonda, “Fungal Diseases Caused by Serious Contamination of Pharmaceuticals and Medical Devices, and Rapid Fungal Detection Using Nano-Diagnostic Tools: A Critical Review,” Curr. Microbiol. 81(1), 10 (2023).
- O.J. Wouters, M. McKee and J. Luyten, “Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018,” JAMA 323(9), 844–853 (2020).
- R. Patel, et al., “A Retrospective Regulatory Analysis of FDA Recalls Carried Out by Pharmaceutical Companies from 2012 to 2023,” Drug Discovery Today 29(6), 103993 (2024).
