Introduction
The US DSCSA was enacted in November 2013 with the aim to improve traceability of drugs from the manufacturer to the end user. There have been various changes to the act and the FDA has revised dates multiple times to allow wholesalers, distributors and dispensers to cope with the changes.
How DSCSA Protects Patients
- Prevent harmful drugs from entering the supply chain
- Detect harmful drugs if they enter the supply chain
- Respond rapidly when harmful drugs are found
When there is credible evidence that points to a product being illegitimate then the FDA expects that Form FDA 3911 is filled out and submitted to the FDA and the training partners within 24 hours.
Latest Events
The FDA recently held a virtual meeting on 16th November 2021 to discuss Enhanced Drug Distribution Security at the Package Level. The aim of this meeting was to take the stakeholder's comments about their current progress, challenges and recommendation. They also discussed some optional non-binding recommendations around aggregation and the secure exchange of information between parties. You can find the details of the meeting here.
Upcoming 2023 deadline
The last extension announced by FDA in October 2020 was to delay enforcement of saleable returns for three years for wholesalers and dispensers. With this extension, all DSCSA regulations will come into full force on 27th November 2023.The FDA has signalled that there would be no more delays of this upcoming deadline.
Timeline of events in DSCSA Implementation
2017 - 2020
Product Identification (Serialisation)
Manufacturers & re-packagers encode product identifiers on prescription drug packages on the smallest individual saleable unit
Verification
- Serialised product can be verified down to the package level using the product identifier
- Saleable returns
- Compliance policies issued that provide additional time
To prevent counterfeiting of saleable returns, wholesalers need to ensure that saleable returns are verified before being introduced to the supply chain again and has an interoperable system that provides interoperable data exchange. This is achieved by initiating a verification request by the wholesaler to the manufacturer to verify the returned products and the manufacturer must provide a response within 24 hours. The verification router service enables real time exchange of this information between the parties.
2023
Enhanced drug distribution security requirements
- All electronic items
- Enhanced product tracing at the package level (including the product identifier)
- Enhanced verification
2023 & Beyond
Enhanced system
- Enhanced drug distribution security across the pharmaceutical supply chain
- Improved inspections and investigations
- Improved data analytics
- Continued compliance and enforcement
DSCSA Goals
Implement interoperable, electronic tracing of products at the package level by 2023 that will:
- Enable secure tracing of product at the package level
- Use product identifiers to verify product at the package level
- Enable prompt response to address suspected and illegitimate products when found
- Improve efficiency of product recalls
- Establish national standards for licensure for wholesale distributors and third-party logistics providers (3PLs)
DSCSA Key Requirements
- Product Tracing
- Verification
- Product Identifier
- Authorised Trading Partner
Authorised Trading Partners(ATPs) under DSCSA
- Manufacturers
- Repackagers
- Wholesale Distributors (WDDs)
- Dispensers (primarily pharmacies)
- Third-party logistics providers (3PLs)
DSCSA mandates that only authorised trading partners (ATPs) may engage in transactions with other ATPs. All parties including manufacturers, distributors, 3PLs and dispensers must be registered as ATPs.
Scenarios where packages are without Product Identifiers
- Excluded products - Not all prescription drugs are required to have a product identifier and are hence excluded.
- Grandfathered - Some products will be in the supply chain before the product identifier requirement took effect.
- Waiver, exception or exemption - Some products were granted a waiver, exception or exemption from the product identifier requirement.
Serialisation Requirements
Serialisation has been made mandatory by the FDA which requires all players in the drug supply chain to adhere to the traceability requirements. Which means that manufacturers, re-packagers, distributors and dispensers must generate, authenticate and verify the serial numbers for all products in the supply chain.
The Standardised Numeric Identifiers (SNI) for the serial numbers must be generated as per the 2009 FDA directive which gives the following guidelines:
- SNIs consist of the 20-character NDC (National Drug Code) and serial number.
- Apart from SNIs batch/lot number, Global Trade Item Number (GTIN), and expiration date are also required.
- The smallest saleable unit needs to be packaged with a 2D Barcode Matrix with human-readable text.
- Shipping cases also require an SNI with a Serial Shipping Container Code (SSCC).
Aggregation and Inference
Aggregation is the process of building a relationship between unique identifiers assigned to packaging containers.
Inference involves examining information for a higher level of packaging to infer information about the next level of packaging and its contents.
3T Documentation
Transactional information
This includes the product information. The following four items are part of the product information
- NDC: [insert product’s NDC]
- SERIAL: [insert product’s serial number]
- LOT: [insert product’s lot number]
- EXP: [insert product’s expiration date]
The DSCSA continues to use The Electronic Product Code Information Services (EPCIS) standard for exchanges.
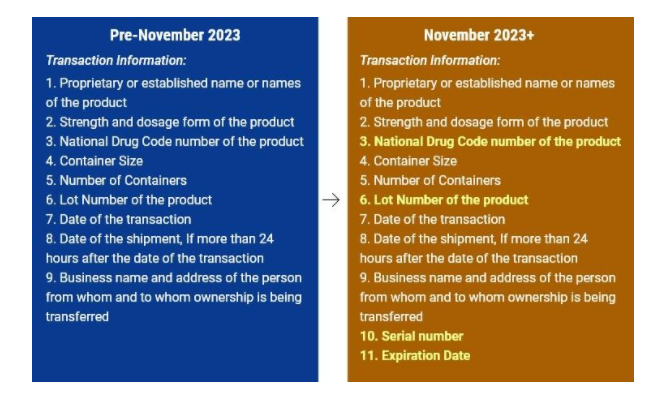
So to summarise, the transactional information including the Product Information should contain.
- The name of the product
- Dosage and strength of the product
- NDC (National Drug Code)
- Serial Number
- Lot number
- Date of transaction
- Date of Shipment
- Date of Expiry
- Container information
- Number of containers
Transactional history
Complete outline of all the transactions the product has gone through in its journey right from the manufacturer to the dispenser which summarises the product's entire supply chain journey and has the required transaction information.
Transactional Statements
These statements identify if the seller is:- Is authorised and registered
- Has received the product from a registered and authorised party
- Did not purposefully change the transaction history
- Did not purposefully ship any ineligible, counterfeit, or suspicious product
- Has acknowledged the transaction statement and information from the previous seller in the supply chain
Disclaimer
This information is being provided ‘As Is’ with no claims of suitability for a particular purpose. It represents just one possible interpretation of information available in the public domain or through membership organisations, and that interpretation is subject to change. This information does not constitute legal advice. Users must refer to the source material for the complete requirements and form their own interpretation before making business decisions. Please use the references below to follow the updates at the source.
https://www.fda.gov/drugs/drug-supply-chain-integrity/drug-supply-chain-security-act-dscsa
