Pharmaceutical macro trends
- High Covid-19 patients requiring hospitalisation may result in an on-going drive to keep other patient conditions managed in a home environment
- GP consultation and diagnosis undertaken remotely likely to become the default method
- Further development of the home-diagnostic / self-medication sectors
- Stay at home culture driving a change in habits
Source: Mintel
Beyond distribution
Surfachem strives to make the difference. Supporting customers beyond supply, Surfachem provides:
- GDP compliant (MHRA audited) / API Certified (FMD) / ISO 9001 certified
- APIs only from fully GMP-approved sources and thorough due-diligence applied
- Secure and transparent GMDP supply chains
- Experts in API approvals, regulatory/quality documentation assessments
- All volumes supplied (R&D, pilot production, commercial quantities)
- Global freight and transportation management to multiple end-user sites
Have you tried?
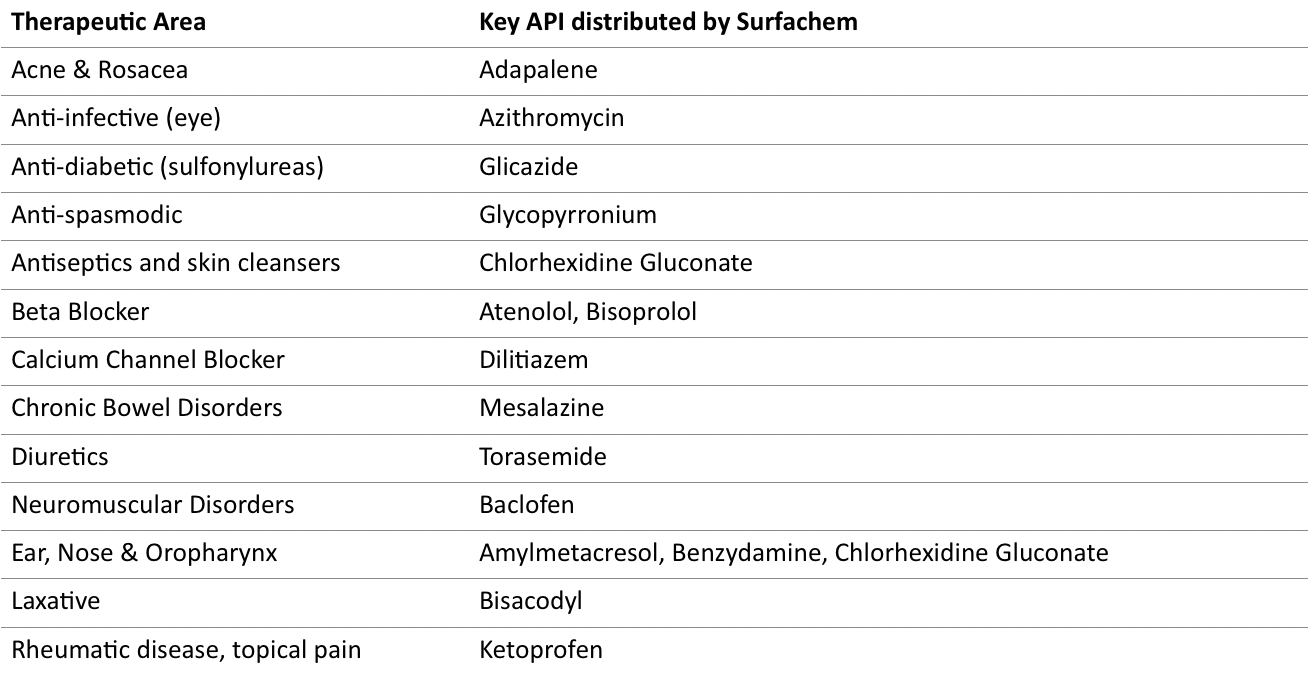
Through an extensive network of suppliers, Surfachem supplies its customers with an extensive range of active ingredients for their current portfolio and new product development requirements. Aiming to provide a full package of regulatory and quality documentation to support APIs, Surfachem is proud to offer its customers relevant and specific product and market information.
The below active ingredients are distributed by Surfachem with CEP/EDMF and EU-GMP certification.
For more information or to request a sample, please contact Surfachem or send an email at info@surfachem.com
Discover more about Surfachem’s expertise by clicking the link below.
