Blockbusters may be in short supply but the latest new chemical entities to receive the EMA’s go-ahead promise to improve delivery, simplify compliance and extend patient outcomes. Dr Sarah Houlton reports.
The death of the blockbuster model for the pharmaceutical industry has been much talked about in recent years, and judging by the list of new products given the go-ahead by the European Medicines Agency (EMA) in 2010, reports of its impending demise are not exaggerated. Few of the new chemical entities (nces) and new biologics are in therapeutic areas likely to see the multi-billion dollar sales that have propped up Big Pharma’s sales figures in the past. The much-heralded patent cliff is now upon us, and with patents running out on the biggest sellers, companies will increasingly have to rely on more niche products for their future profits.
That said, the list of new approvals contained several interesting new products with good potential for patients. Cardiovascular disease remains a big killer, despite the numerous blockbuster drugs that have improved outcomes by lowering blood pressure and reducing cholesterol levels. One condition that is still undertreated is atrial fibrillation (AF), the most common form of abnormal heart rhythm, and with a recent metastudy implying that patients with AF appear to be more likely to develop dementia, being able to treat it is set to become more important.
Merck’s vernakalant (Brinavess) – licensed from Cardiome Pharma – is such a drug. The antiarrhythmic drug blocks potassium channels in the atrium, as well as sodium channels, but unlike other potassium channel blockers its potency increases as the heart rate goes up. This renders it more effective in patients with faster heart rates, and in a Phase III trial it was shown to be significantly better than the existing drug amiodarone at converting the abnormal heartbeat into normal sinus rhythm within 90 minutes of treatment starting. The drug has been licensed as an intravenous infusion but the company is also working on an oral formulation that would potentially be more acceptable to patients and increase its use.
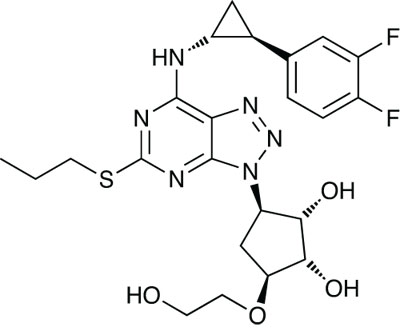
Ticagrelor (Brilinta)
A new antiplatelet drug, AstraZeneca’s ticagrelor (Brilinta), was given the go-ahead late in the year. The antiplatelet market is a big one – sanofi-aventis’ clopidogrel (Plavix) has been near the top of the bestseller list for some years – but there is still potential for new drugs with different mechanisms of action. Like clopidogrel and related thiopyridine drugs, it blocks the P2Y12 subtype of ADP receptors but it has a different binding site and its blocking action is reversible. A further advantage is that it does not need activating in the liver.
Trials showed it to have a faster onset and shorter duration of action than clopidogrel, so it has to be dosed twice a day rather than once, which may impact on patient compliance. However, the compound’s reversibility and shorter half-life gives it some advantages if bleeding occurs. In a Phase III trial, subjects given ticagrelor were less likely to die from heart attack, stroke or vascular events but non-lethal bleeding was slightly more frequent.
Reducing the number or frequency of doses is a good way of increasing compliance, and one way that this can be done is by combining drugs that are commonly taken together into one pill. The new hypertension treatment Twynsta from Boehringer Ingelheim is just such a product – it combines its own angiotensin II antagonist telmisartan with Pfizer’s calcium channel blocker amlodipine in a single dosage form. Hypertension is commonly treated with more than one drug and this is the latest in a collection of combination therapies to reach the market.
Hereditary angioedema is a far less common condition and Pharming Group’s orphan medicine conestat alfa (Ruconest) is designed to treat it. Patients with the condition suffer from swelling that can occur anywhere in the body, leading to discomfort and pain. There is no single cause but the new injectable drug is designed to treat attacks where the angioedema is linked to low levels of the protein C1 esterase inhibitor. This protein is involved in the control of the complement and contact proteins in the blood, and angioedema results when this system’s activity is too high. Conestat alfa is a recombinant version of the C1 esterase inhibitor protein that inhibits this activity. It is extracted from the milk of genetically modified rabbits.
Huge amounts of research effort are being put into hunting for new treatments for cancer, but only three new anticancer products were approved last year, two of them from GlaxoSmithKline (GSK). The first of these, ofatumumab (Arzerra), licensed from Genmab, is designed to treat chronic lymphocytic leukaemia that is refractory to other treatments. It is a human monoclonal antibody generated using transgenic mouse and hybridoma technology, and is made via a recombinant murine cell line by cell cultivation. It binds to both the small and large extracellular loops of the CD20 protein on B-cells, and inhibits early activation of the B-cells.
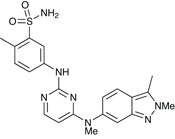
Pazopanib (Votrient)
GSK’s second new anticancer agent, pazopanib (Votrient) is licensed to treat advanced renal cell carcinoma. The small molecule drug is a tyrosine kinase inhibitor that is active against the cytokine vascular endothelial growth factor, or VEGF. By blocking VEGF, angiogenesis – the process by which the tumour develops blood vessels that embed into the body’s vascular system – is inhibited. In Phase III trials, it was shown to reduce the risk of tumour progression or death by 54% compared with placebo, increasing progression-free survival from 4.2 to 9.4 months. An increase was even seen in pretreated patients, from 4.2 to 7.4 months.
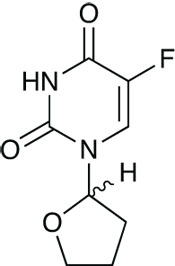
Tegafur (Teysuno)
The third new anticancer agent, Taiho Pharma’s Teysuno, is an orphan drug designed for use in advanced gastric cancer. Given in combination with cisplatin, its active is the 5-fluorouracil derivative tegafur. The capsules also contain two compounds that modulate 5-FU metabolism, gimeracil and oteracil. While the chemotherapy regimen of 5-FU plus a platinum drug has been a mainstay of the treatment of numerous cancers for many years, it has to be dosed by continuous infusion into a central venous catheter. This combination renders it orally available and hospital admission is not essential, as is the case with the parent drug. Trials have shown it to be non-inferior to 5-FU and the oral dosing is a real advantage.
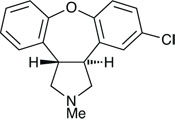
Asenapine, created by Organon
There are many areas of neuroscience where more effective drugs are needed, providing a great deal of market potential, and two new CNS agents were approved last year. One of these, asenapine (Sycrest), developed by Organon but now owned – after two company acquisitions – by Merck, is indicated for the treatment of moderate to severe manic episodes in patients with bipolar disorder. As with other atypical antipsychotics the precise mechanism of action is unknown, but it is thought to act as an antagonist at the D2 and 5-HT2A receptors.
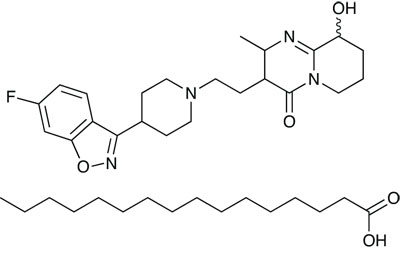
Paliperidone palmitate
The second new CNS agent is Janssen-Cilag’s paliperidone palmitate (Xeplion), for the treatment of schizophrenia. It is actually a prodrug formulation of the existing drug paliperidone (Invega), which itself is a metabolite of the long-standing antipsychotic drug respiridone. Like asenapine, it is thought to antagonise the D2 and 5-HT2A receptors. But unlike the parent compound, which is formulated as extended release tablets, it is a prolonged release injection, and is indicated for maintenance treatment in schizophrenic patients whose symptoms have been stabilised by paliperidone or risperidone, and in some cases where the psychotic symptoms are less severe, or in patients who have not been previously stabilised. As it needs to be administered only once a month, it has potential to significantly improve compliance in a patient group where this is often a substantial problem.
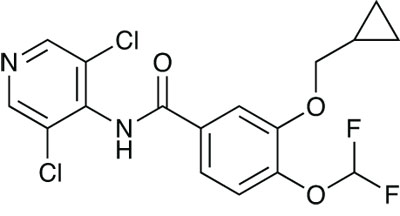
Roflumilast (Daxas)
Chronic obstructive pulmonary disease is another therapeutic area that is inadequately served by existing drugs, and thus also retains great potential for new drugs. Nycomed’s roflumilast (Daxas) was approved for severe COPD associated with chronic bronchitis. It is a selective and long-acting inhibitor of phosphodiesterase-4, an enzyme that is prevalent in immune cells, and which breaks down cyclic adenosine monophosphate. This causes inflammatory effects, and preventing this breakdown improves the symptoms of inflammatory diseases, such as COPD, by relaxing the smooth muscle in the respiratory system.
As so many of the big therapeutic areas are already well served by medicines, an increasing proportion of the drugs now being approved are designated as orphan. Rare diseases are less appealing to pharma companies because of the limited size of the patient population, so the regulatory authorities make it easier to develop drugs for these areas where treatment options are limited, if available at all.
orphan products
Several orphan drugs were approved last year, in addition to those already described. One of these, Shire’s velaglucerase alfa (Vpriv), was particularly important. It is designed to treat Gaucher’s disease, a hereditary enzyme deficiency disorder where lack of glucocerebrosidase leads to the accumulation of glucocerebroside in the white blood cells and organs such as the liver, kidneys, lungs, spleen, brain and bone marrow. Painful symptoms include an enlarged liver and spleen, neurological problems and a distended abdomen. The replacement enzyme imiglucerase (Cerezyme) has been available from Genzyme for some time, but manufacturing problems at the company’s Allston, Massachusetts plant meant that the drug was in short supply, so the approval of Shire’s recombinant product gave patients an alternative that meant their symptoms could continue to be treated.
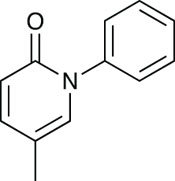
Pirfenidone (Esbriet)
Until last year’s approval of Intermune’s pirfenidone (Esbriet), there was no effective treatment for idiopathic pulmonary fibrosis. Patients with this condition develop scarring in their lungs and the air sacs are slowly replaced by thick, fibrotic tissue. This irreversible process causes the tissue to lose its ability to transfer oxygen into the bloodstream, leading to a shortage of breath, weakness, fatigue, weight loss, cough and discomfort in the chest. Ultimately, it is fatal. The new drug slows cell proliferation by inhibiting several growth factors: fibroblast growth factor, epidermal growth factor, platelet-derived growth factor and transforming growth factor beta 1.

Researchers at the Novartis Institutes for BioMedical Research (NIBR) centres in Cambridge, Massachusetts, Basel, Switzerland, and Shanghai, China develop small molecule drugs and antibodies.
Image source: Novartis
Respiratory tract infection is a real problem for patients with cystic fibrosis, particularly with Pseudomonas aeruginosa. Another orphan product, the TOBI Podhaler from Novartis, is designed for the long-term management of this infection risk in cystic fibrosis patients over the age of six. The active ingredient is the aminoglycoside antibiotic tobramycin. This antibiotic has been used for a while in this indication, but it had to be delivered by nebuliser, which gave sub-optimal efficacy. The new product is a dry powder formulation, created using the PulmoSpheres technology developed by Alliance Pharmaceutical. It involves a lipid-based emulsion of the active ingredient being incorporated into hollow, porous dry powder spheres, with a diameter of about 1–5mm. In clinical trials, this inhaler formulation reduced patients’ treatment burden by significantly shortening the drug’s administration time, and reducing the need for nebulisers.
Tobramycin, being used by Novartis
The final orphan product given the go-ahead by EMA last year was Laboratoires CTRS’s Orphacol hard capsule formulation of cholic acid for the treatment of inborn errors in primary bile acid synthesis resulting from a deficiency in one or other of the enzymes 3b-hydroxy-d5-C27-steroid oxidoreductase or d-4-3-oxosteroid -5b-reductase. Cholic acid is naturally present in bile acid, and the oral formulation replaces the missing bile acids, providing their physiological and metabolic regulation functions that are otherwise absent. As well as restoring biliary secretion and biliary elimination of toxic metabolites, it inhibits the production of hepatotoxic bile acid metabolites by giving a negative feedback on cholesterol 7a-hydroxylase, the rate-limiting enzyme in bile acid synthesis. It also improves nutrition by improving the absorption of fats and fat-soluble vitamins in the intestines.
Not all unusual diseases class as orphan, however. A new product from Pfizer, Xiapex (collagenase clostridium hystoliticum), was developed to treat Dupuytren’s contracture. Patients with this condition find that their fingers bend towards the palm of their hand, and they are unable to straighten them fully, with the ring and little fingers being the most badly affected. While it is usually painless, it is slowly progressing and has an impact on the hand’s normal function. It can be corrected by surgery, but complications are common. The new drug is an injectable collagenase extracted from the bacterium Clostridium histolyticum. The drug is injected into the cord in the palm of the hand formed by shortened tissue that restricts the tendons into the finger, and dissolves it.
A novel presentation of an old drug from Allergan was also approved – the steroid dexamethasone under the brand name Ozurdex as an intravitreal implant for the treatment of macular oedema resulting from retinal vein occlusion. The implant itself is made from the Novadur solid polymer delivery system, which contains poly(D,L-lactide-co-glycolide) PLGA intravitreal polymer matrix, which slowly degrades to lactic acid and glycolic acid. The rod-shaped implant is preloaded into a single-use applicator for injection directly into the eye. The corticosteroid it contains suppresses inflammation by inhibiting several inflammatory cytokines, which reduces oedema, fibrin deposition, migration of inflammatory cells and capillary leakage.
vaccines
The continual potential for pandemic flu has led to three new flu vaccines. One of these, Medimmune’s Fluenz, is specifically designed for use in children. The other two – GSK’s Pumarix and Novartis’s Aflumov/ Prepandemic – are mock-up pandemic flu vaccines that are ready for the insertion of the relevant pandemic virus strain once it appears. Pre-approving vaccines in this way will dramatically reduce the length of time between a pandemic emerging and a vaccine being available to prevent it, and while they are not the final product, they are an important element of pandemic preparedness.
Finally, one new imaging agent was also on the list of new products last year. Regadenoson from Gilead, marketed as Rapiscan, is an A2A adenosine receptor agonist which acts as a coronary vasodilator. It has a rapid onset of action, and at two to three minutes, its half-life is ideal for radionuclide myocardial perfusion imaging – adenosine itself has a half-life of just 30 minutes.
Fluenz production in Philadelphia, US, where the drug is marketed by MedImmune under the trade name FluMist
