Adeno-associated viruses (AAVs) have emerged as one of the leading gene therapy systems because of their ability to transport beneficial genetic material into patient cells with minimal pathogenicity and a low risk of mutagenesis.
They have been a critical component in nearly 250 clinical trials, including some that have received US FDA approval, such as the retinal therapy Luxturna. With more than 400 ongoing gene therapy trials in the United States alone, researchers learn more every day about the best delivery systems for these therapeutics.
To create AAV-based gene therapies, developers first produce recombinant AAVs that contain the desired DNA sequence with the cell as the host. Then, by extracting and purifying the virus particles, the host cell contains the new sequence with no impurities or contaminants.
But, even methods as promising as this one are not perfect.
One of the primary challenges with AAVs is making sure that the viral vectors delivered to the patient are pure, safe and effective.
Otherwise, transporting the vector into the patient’s cell could put them at risk. To ensure the safety of AAVs, manufacturers must put rigorous quality control measures in place.
Testing for contaminants and determining the proper viral titre is crucial, especially as production is scaled, which often complicates things. Here, we discuss the five major challenges that we’ve observed in AAV development.
Challenge one: Mycoplasma contamination
If bacteria of the genus Mycoplasma contaminate therapies, it puts patients at risk of respiratory illness, making it an especially important impurity to watch out for.
Although the risk is significant, however (30% of cell lines are contaminated), these bacteria are hard to detect because they are extremely small and difficult to see with a standard light microscope.1
Also, they are resistant to the beta-lactam antibiotics typically used to maintain cell lines. Alternative quality control methods such as cell culture procedures, real-time PCR (RT-PCR) and Droplet Digital PCR (ddPCR) technology should instead be used to ensure AAV quality.
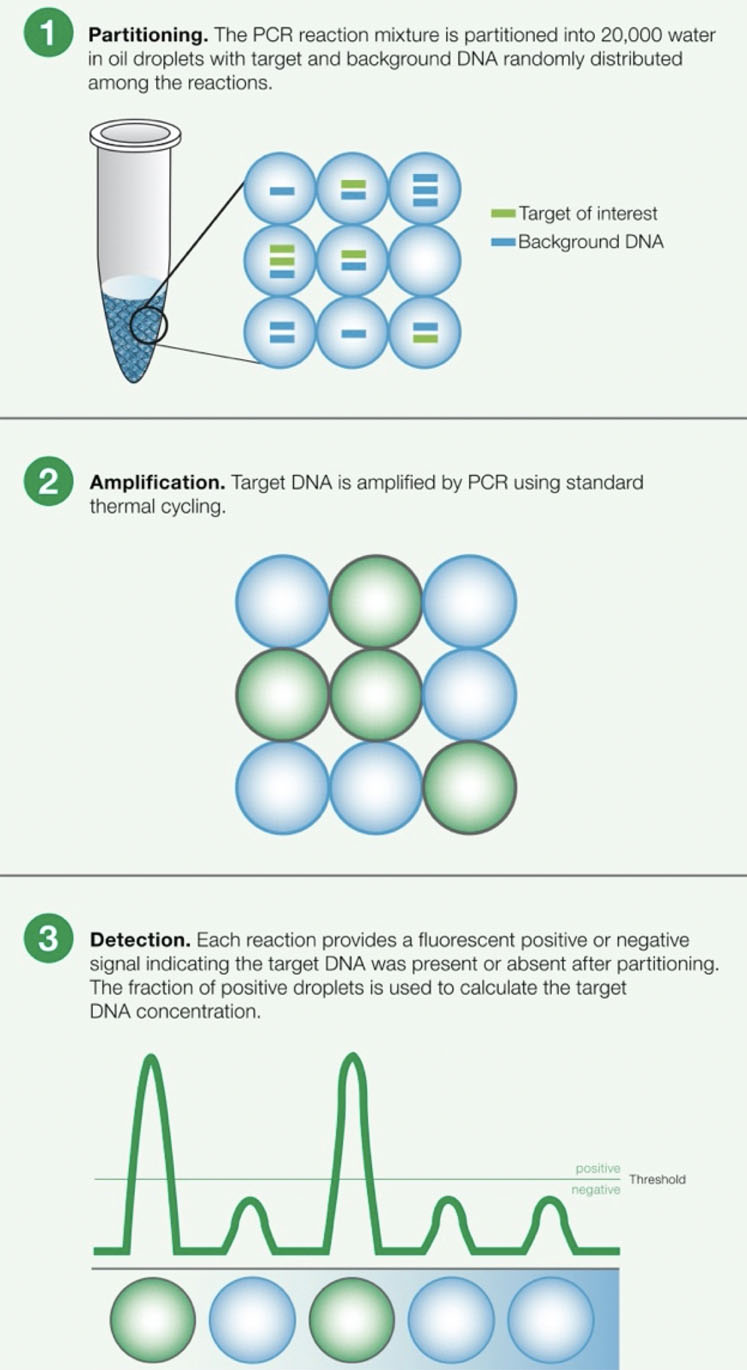
Figure 1: Principles of droplet digital PCR (ddPCR)
Although RT-PCR is one of the most common tools used for this purpose, it may not be the best. This technology relies on a standard curve generated by counting the number of cycles it takes for a sample to fluoresce at a certain threshold.
This approach can introduce many human errors, causing results to vary by up to a factor of two.2 Many experts turn to ddPCR tools instead when attempting to detect Mycoplasma because it provides absolute quantification (Figure 1).
Because of this capability, ddPCR technology can precisely quantify Mycoplasma concentration rather than relying on a standard curve, thus providing a more sensitive measurement with less interference from secondary DNA structures or host cell contaminants compared with RT-PCR.
Challenge two: empty capsids
Another common impurity that arises in AAV development is empty or partially full capsids. Capsids that do not contain the intended sequence reduce the effectiveness of the therapy.
Although they may not pose a serious risk to the patient, these capsids can cause therapeutic delivery issues. Because of the reduced effectiveness, the patient needs a higher dose than would otherwise be required.
With a higher dose, it can be a challenge to deliver the therapy to certain parts of the body — smaller areas such as the central nervous system can be tough to reach. To prevent these difficulties, manufacturers must monitor and remove empty and partially full capsids to ensure that most or all of the capsids provide the intended therapy.
Challenge three: vector concentration
To ensure the effectiveness of gene therapy, the vector should be present at the intended concentration. However, the current standard processes used to grow these vectors for upstream bioprocessing yield concentrations that are lower than what is needed to treat patients effectively.
For reference, standard approaches yield concentrations of up to 2 x 1011 vector genomes per mL, whereas effective doses are 1 x 1014 vectors per mL.3 Therefore, the virus needs to be concentrated between 100 and 10,000 times. This process also increases the concentration of the host cell contaminants in the batch.
In turn, this escalates the need for effective quality control to detect and remove contaminants.
Challenge four: immunogenic protein impurities
Although many scientists believe that AAVs are less immunogenic than other delivery systems because they do not contain engineered lipids or other chemical compounds, the capsid protein itself can elicit an immune response.
If that happens, the therapy effectiveness may be reduced. If the patient has been exposed to an AAV before, they may naturally have a pre-existing immune response.
More research is needed to understand the role that empty capsids play in creating this potential response — which is another reason why we need sensitive tools that can detect and quantify these capsids.
Challenge five: oncogenic host cell DNA
In addition to external contaminants and issues with the viral vectors themselves, impurities can arise from the cells used to grow the vectors. These cells may contain oncogenic DNA sequences from the tumorigenic cell lines used to grow the vectors.
These residual sequences could potentially make their way into the final gene therapy product. To solve this problem, developers should test for oncogenes in therapeutic batches.
They should also perform separate tests to determine if their therapy’s genetic sequence incorporation is likely to cause cancer. Unfortunately, once an incorrect sequence is packaged into the viral particle, it is challenging to remove because the capsid shields the DNA from nucleases.
Addressing quality control challenges in AAV development
There are a whole host of impurities and contaminants that can complicate AAV development. As this therapeutic delivery system matures, understanding and implementing the best quality control measures will help us deliver on the promise of AAVs.
Tools such as qPCR and ddPCR technology are effective methods to detect many of these impurities, such as Mycoplasma, vector concentration and oncogenic DNA. As previously discussed, ddPCR technology may be the more sensitive of the two options.
One study found that ddPCR assays were four times more effective than qPCR at detecting single-stranded AAV genomes.4 Another found that ddPCR systems could detect A. laidlawii standards in suspension at one colony forming unit/mL — even when RT-PCR produced a negative result (Figure 2).5
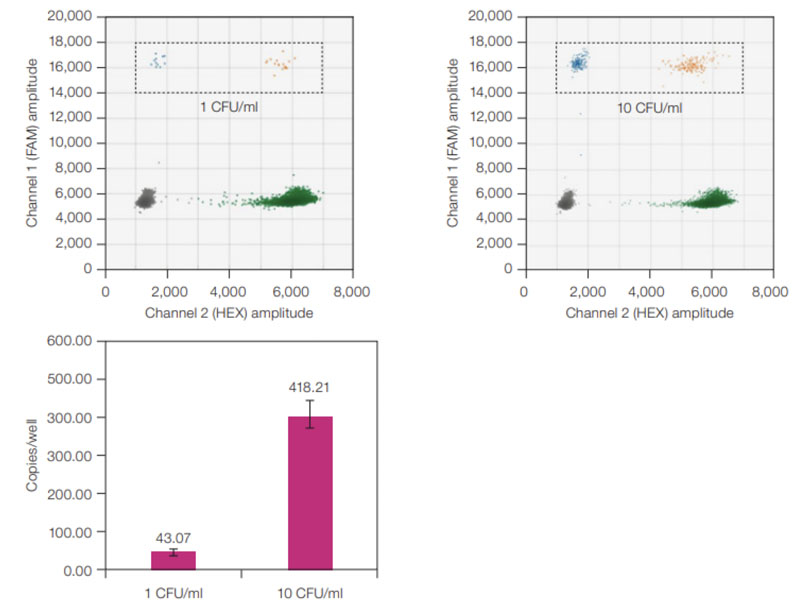
Figure 2: ddPCR sensitivity for A. laidlawii standards. ddPCR 2-D plots (top) with A. laidlawii-positive droplets highlighted in the black, dashed rectangles; average copies/well values (bottom) for 1 and 10 CFU/mL A. laidlawii standards. The ddPCR LOD was 1 CFU/mL because all ddPCR samples tested positive with ≥1 FAM-positive droplet/well. Error bars represent standard deviation (n = 12)
When looking for contaminants in gene therapies, this level of sensitivity and precision is critical because it can help developers to assess treatment potency and determine correct dosages while ensuring the therapy is safe and effective.
Gene therapies come with unique concerns compared with standard drugs. With a traditional therapeutic, developers can more easily predict an active ingredient’s quantity versus a contaminant.
But because virus-mediated gene therapies cannot be as easily controlled during manufacturing — because they are derived from natural and biological processes — there is more uncertainty about what exactly is in each batch.
If we want to realise these the full potential of these therapeutics, it will be essential to make sure our quality control processes are thorough and robust.
References
- www.sciencedirect.com/science/article/abs/pii/S0176672488801767?via%3Dihub.
- https://blog.addgene.org/droplet-digital-pcr-for-aav-quantitation.
- www.semanticscholar.org/paper/Downstream-bioprocessing-of-AAV-vectors%3A-industrial-Hebben/bbf9c9436e5970499eb75bd2a33888ccec68bd84.
- www.cellandgene.com/doc/viral-quantification-adeno-associated-virus-vector-genome-titer-assay-0001.
- www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_7427.pdf.
