Being frozen, pressurised and heated sounds like torture! In fact, though, this is a routine process that’s regularly employed to manufacture biopharmaceuticals and many other drugs, providing a gentle method to remove excess water. The result is the required active ingredient in form of a fine powdery substance.
Freeze-drying, alsoknown as lyophilisation, is often used within the greater pharmaceutical industry to convert antibiotics, hormones, antibodies, RNA-based vaccines and many other pharmaceutical drugs into a storable, solid powder form (cake).
“Most patients receive these drugs in liquid form rather than as tablets as the sensitive molecules are not particularly stable in liquid form. They can change in structure and lose their effectiveness,” explains Stephan Reuter, Managing Director of Optima pharma GmbH (Gladenbach, Hesse, Germany). The company specialises in the development and production of freeze-drying systems.
Gentle evaporation of frozen water
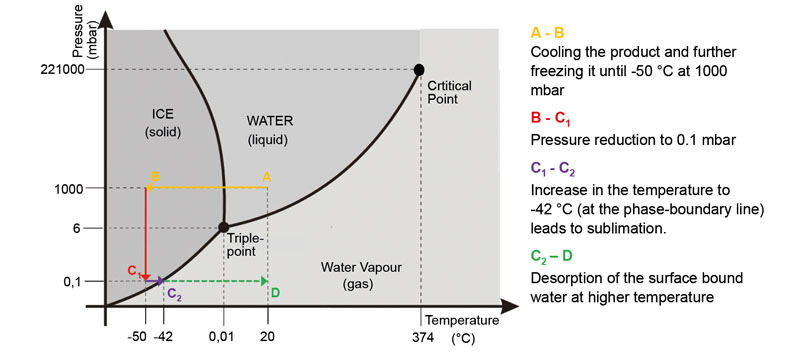
What makes freeze-drying a gentle process? A key aspect is the frozen water that evaporates under vacuum (sublimation) while the temperature is gradually increased. Ideally, only the required product remains (in the form of a fine powder).
Another way to extract a dissolved substance from water is familiar to anyone who cooks: boiling water until it has evaporated. However, this latter approach is anything but gentle. The problem here is that the product will be irreversibly damaged by sustained high temperatures.
In addition, not only will the water disappear, but the required product might change into a gaseous state and also be lost. “Freeze-drying, by contrast, is a safe method and proven to convert even sensitive biopharmaceuticals into a stable and storable product,” Reuter says.
Biopharmaceuticals: freeze-drying is preferred
Owing to factors such as the coronavirus pandemic, as well as the growing number of biological pharmaceuticals and their transport, demand for freeze-drying continues to increase. Optima Pharma has doubled its production area in Gladenbach where its equipment is manufactured. Furthermore, the number of employees has almost doubled since 2014 and the company’s export share is more than 85%.
Reuter is confident that this positive trend will continue. “Currently, approximately one fifth of the top 100 pharmaceuticals are freeze-dried and, for biologics, that figure rises to almost half,” Reuter says. As an engineer, Reuter has two decades of professional and management experience in the pharmaceutical industry, providing some insight in terms future product development.
“The demand for freeze drying has also increased the requirements for the lyophilization process itself. As a result, we’re developing innovative and sustainable solutions to meet these needs.”
Peptides are promising pharmaceutical drug candidates
The lyophilisation of peptides requires complex production processes and special expertise. Peptides are small proteins that consist of several amino acids linked by peptide bonds. They have numerous functions; some act as messenger substances in plants and others as hormones in the human body.
Owing to their properties, there are numerous potential applications for these small proteins in molecular biology, immunology and (bio)medicine. Peptides offer new opportunities for cancer patients, the chance for individualised vaccines and, as such, promising strategies for effective therapies.
“Peptides are highly effective and they exhibit high selectivity and specificity with regard to their biological targets,” Reuter explains. “Peptides thus offer a promising prospect for novel drug designs and are of great value.”
The fine art of freeze-drying
Because of their great versatility, freeze-drying equipment must be designed to meet the needs of peptide production, the final stage of which involves solid-liquid separation. “The freeze-dried product or cake is obtained at our customer’s facility in containers that resemble baking trays,” Reuter says.
The final stable peptides are then available in milligrams to kilograms as a fine powder and used as active ingredients for the production of various pharmaceuticals.
“There is no off-the-shelf freeze-dryer that can accommodate every requirement. We have to adapt the equipment to the particular pharmaceutical product, according to customer needs requirements and on-site conditions. This is our expertise as a special equipment manufacturer,” Reuter says.
The company is also involved in a promising new area of development: controlled nucleation. The focus is the difficult-to-control freezing process, especially for highly purified products. An irregular freezing process has a corresponding effect on the ice structure.
However, controlled nucleation is a process that solves this problem: additional pressure is created in the system and released quickly, resulting in formation of very homogenous ice crystals and, at the same time, accelerating the drying process.
Energy saving
Shorter process times reduce material consumption and, above all, save energy. “Despite the benefits offered by freeze-drying, it’s also a very energy intensive process … and correspondingly costly,” explains Reuter.
“After all, the stainless-steel systems, which can weigh up to 30 tons, must first be sterilised with steam at up to 130 °C, cooled to –70 °C, placed under vacuum and then reheated to 130 °C again to start the process. This is the only way to achieve the desired temperatures inside,” Reuter adds. “As a result, drug manufacturers are often reluctant to consider this technology for their products.”
However, refrigeration technology and new high-performance cooling agents can yield significant savings: using a dedicated refrigeration system lowers energy consumption. In addition, new innovative cooling agents also save 10–20% in energy costs.
Considering the savings derived from shorter process times, freeze-drying systems consume up to 25% less energy than before these measures were implemented.
Focus on medical trends
Optima pharma is also working to establish alternative cooling agents and to create more sustainable and environmentally friendly technologies. New trends in the pharmaceutical industry are an additional reason for new developments, such as personalised medicines, mRNA and cell and gene technologies.
“These changes influence how drug production facilities operate; they’re tending to require fewer large systems and focus on making smaller batches with smaller equipment,” Reuter says. “As such, biopharmaceutical drug companies — and even hospital pharmacies — want (multiple) freeze-dryers with a reduced footprint.”
Today’s freeze-drying process has become much more energy efficient and increasingly diverse compared with just a few years ago. Optima pharma has made it a priority to keep an eye on evolving industry trends and to develop new process technologies accordingly, thus making an important contribution to innovative, high-quality pharmaceuticals.
