New plasma processes have been developed to improve the surface energy performance of a pMDI canister. Richard Turner of Presspart Manufacturing explains how they can improve drug stability and drug delivery
Pressurised metered dose inhalers (pMDIs) are commonly used to deliver drugs for treating respiratory and nasal disorders. The drugs are administered by aerosol, in suspension or solution, with a liquefied gas propellant. For more than 50 years, chlorofluorocarbons (CFCs) were the propellants of choice, but these were phased out and finally banned at the end of 2008, in line with the Montreal Protocol.1
Replacement propellants have been developed over the past two decades based on hydrofluoroalkanes (HFA), specifically HFA 227 and HFA 134a. These substances are not ozone-depleting, they are also non-flammable and chemically inert, making them ideal candidates for use in medical products. However, some properties of these compounds are substantially different from those of the CFCs traditionally used in MDIs.
The surface properties of a device can have an important effect on the device’s interactions with its most immediate environment and substances with which it comes into contact. As a result, the device’s surface chemistry has a vital role in the surface functionality and, therefore, overall performance of the device and drug.
When HFA-MDI drug formulations are in suspension, interactions with the canister substrate can cause deposition of the drug on the canister walls or on exposed surfaces of the valve components. Interactions with solutions more commonly cause degradation, resulting in increased impurity levels. In both cases the interaction leads to a reduction in the drug content in the formulation, resulting in the patient receiving less than the prescribed dose.
Applying a suitable surface coating to the MDI components improves the stability of the formulation as well as the product performance, and helps to extend the product’s shelf life. A range of coatings have been developed that can be applied to both the canister and valve components to protect the contents from deposition and degradation.
Commonly used coatings include barrier coatings, such as anodisation of the canister, to change the surface characteristics and ultimately act as a protective barrier for sensitive formulations.
Various low surface energy coatings are available for suspension formulations. For example, a surface treatment has been specially developed for deep-drawn anodised 5052 aluminium canisters and is suitable for budesonide HFA; several new coating compounds such as E3 and E5 acrylic polymers have been developed that prevent certain HFA-containing drug formulations (e.g. Ventolin) from interacting with the MDI and adhering to canister walls.
Fluorocarbon polymers are commonly used to coat the interior canister surfaces to eliminate adhesion or deposition of albuterol on canister walls; albuterol is widely used with MDI drugs, particularly beclomethasone dipropionate. Fluorocarbon polymers used in coatings are commonly made from multiples of one or more of a variety of monomers; particularly preferred coatings tend to be pure perfluoroalkoxyalkylene (PFA), and blends of polytetrafluoroethylene (PTFE) and polyethersulphone (PES), due to their relatively high ratios of fluorine to carbon. In addition, coatings that combine fluorocarbon polymers with non-fluorocarbon polymers (e.g. polyamides) are used for certain formulations to improve adhesion of the coating to the canister walls; other coating types include epoxy-phenol resins or even a thin film of glass.
coating techniques
Standard metal coating techniques can be used to pre-coat the metal substrate and cure it, prior to shaping the metal into the components (e.g. through deep-drawing or extrusion). This pre-coating method has the advantage of being well suited to high volume production. Other coating techniques include spraying the insides of preformed cans, dipping, or electrostatic dry powder coating, followed by curing.
Many of these processes require high temperatures (up to 400°C when curing), which can create additional costs and complications. Furthermore, only the most robust canisters (i.e. those produced through deep-drawing) should be subjected to such high temperatures, as less robust canisters can become unrolled or suffer other morphological changes under these conditions.
More recently, gas plasma-based processes have been developed to modify and improve the surface energy performance of a pMDI canister. Gas plasma processing is an industrial technique that is carried out in a vacuum to coat a wide range of substrate materials. The process involves constant or pulsed excitation of gas by either Radio Frequency (RF) or microwave field to produce an energetic plasma.
The process creates an ultra thin layer that protects against degradation, deposition and corrosion. It is a low temperature process (<75°C for metallic substrates and <45°C for polymeric substrates), and is ideal for uniform treatments of components with complex shapes, including small components in large volumes. The coating adheres well to the component substrate, because the plasma process cleans the component surface while in the vacuum, resulting in an ultra-clean substrate-coating interface.
plasma coating techniques
Using gas plasma to tailor the surface chemistry has the advantage of providing uniform surface treatment without changing the properties of the bulk material. The process can be used to change the outermost layers of the material only, without polymerising a coating, resulting in modifications to the functional chemistry. These modifications can be used as a stand-alone or with the addition of a subsequent surface coating through a single process cycle, depending on the application and desired properties.
Plasma processing of MDI canisters can bring multiple benefits to the MDI performance, helping to reduce drug deposition and also to improve the stability of formulations where interactions with the aluminium substrate would lead to product degradation and reduced shelf life. However, plasma processing for MDI canisters needs to be highly controlled to ensure complete consistency of treatment and uniformity of coating to the internal walls of the canisters.
Plasma chemistry is critical to the performance of the coated canisters – the right choice of precursor chemistry enables a robust process with excellent performance. A variety of plasma treatments have been tried in the past, including single and dual layer technologies with a range of monomers, but these have failed to penetrate the market due to poor scalability and cost viability.
However, alternative developments have become available that make plasma a real choice for pMDI cans. A cost-effective process has been established using an optimised plasma chemistry consisting of an intrinsically robust monomer, highly ionised to form a high crosslink density. The ultra-pure gases and monomers do not contain any solvents, so do not produce any waste by-products. The result is a coating technology without the extractable issues potentially encountered with some polymer systems.
more precise coating
It is critical that plasma processing achieves complete and consistent coating across the entire surface of the inside of the canister. Traditional plasma processes, RF or microwave, are particularly difficult to control when internal surfaces are to be treated. Poor penetration of plasma ions with low energy results in non-uniform, thin or porous coatings with poor performance. Increased ion energy to aid depth of can penetration gives rise to ion etching at the can neck and a more ‘line of sight process’.
This partial ‘line of sight’ process leads to non-uniformity/thickness variation in such geometries (see Fig 2a); for thin nanometre coatings on pMDI cans this is observed as striations in colour or colour bands down the can. With the best compromise the coating builds up around the canister lip, throat and base, with depletion at the rim, shoulders and can corners.
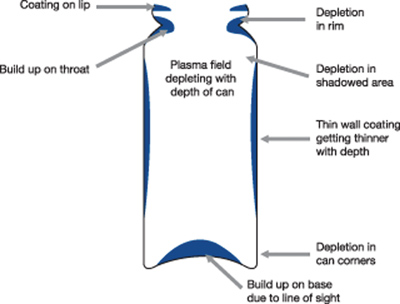
Figure 2a: Traditional plasma processing does not ensure a uniform coating to the internal walls of the canister
More recently, an improved process has been developed that eliminates the issues associated with typical plasma system designs. Using proprietary gas/monomer delivery configurations and electric field control (designed specifically for can coating geometry), uniform coatings can be deposited.
Dedicated system design configurations mean constant, high deposition rates with extreme reproducibility in terms of coverage, chemical speciation and product performance (see Fig 2b). The unique combination of process equipment design and precursor monomer means the technology is now scalable to handle the throughput and commercial demands of the pMDI world market.
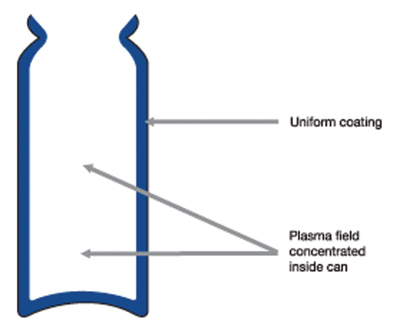
Figure 2b: The new plasma process gives a uniform coating to canisters
This process has been used to develop several different plasma coating options that successfully prevent drug deposition on the can walls, and prevent drug degradation in solution or suspension. Examples include surface treatments for budesonide, formeterol, fluticasone propionate and beclomethane dipropionate, among others.
In conclusion, gas plasma processing offers considerable advantages in the coating and treating of pMDI canisters for improving the stability of the formulation and extending product shelf life. In addition, the ability to plasma process high volumes of the canisters fulfils the high volume demand from the pMDI market.
reference
1 Montreal Protocol on Substances that Deplete the Ozone Layer
