Clinical trials are usually tendered for and awarded on cost. Paul Naybour, md at Parallel Project Training, and Roger Joby, project management consultant at 1to1to1, argue that the focus should be on value rather than cost
A recent presentation to the Pharmaceutical Contract Management Group (PCMG) conference, entitled ‘Value Management – It’s not all about the money’, held recently in Sitges, Spain, a comparison was made between the practice in clinical research and that in other sectors.
It is interesting to compare the highly competitive commodity procurement practice in most of the clinical research industry with the more collaborative approached used in some partnership projects. It is important for project managers in clinical trials to focus on a collaborative approach to define and meet the business needs, focus on value rather than cost, consider options using a structured approach and prevent unnecessary expenditure and waste.
The typical clinical research procurement process has a major limitation because those involved in the execution of the project are not often involved in the specification of the protocol. It usually runs as follows:
Concept: Early in the trial the sponsor defines the objectives for the project. Often without input from either the in-house or outsourced research team, these objectives can be arbitrary and unrealistic.
Design: The sponsor (a pharmaceutical company) prepares a protocol for the trial. The feasibility study is sometimes carried out in isolation from the clinical research organisations (CRO) that are going to complete the study or the CRO is asked to include feasibility as part of the selection process. Thus many assumptions about the best way to implement the protocol are made with no, or insufficient, input from the CRO.
The assumptions made at this point may not reflect the most practical approach to key areas, such as recruitment of patients, selection of centres etc., which can have a profound impact on timelines and costs at a later stage.
Procurement: CROs rarely have longer than two weeks to tender for the work, following receipt of a request for proposal (RFP). The contracts are usually some form of fixed price. The aim of the CRO at this point is to win the contract and limit the liability. Very little time exists to do any proper feasibility study into the proposed protocol and the CRO is in ‘sales mode’ so is unlikely to be honest about a poor quality approach. The more likely strategy is to ensure that the scope is tightly defined and CRO is protected from the weakness in the protocol by clearly defined assumptions that pass the risk back to the sponsor.
At the end of this phase the sponsor will select the best supplier. This may well be the cheapest. One reason it may be the cheapest is because they have successfully transferred the risk back to the sponsor.
Implementation: During the implementation phase virtually all project plans need to be amended, often in response to a more realistic assessment of site set-up times, patient recruit-ment etc. The CRO will expect the sponsor to cover the costs of these amendments.
Many of these changes could have been readily foreseen in the feasibility phase if the people involved in completing the study were involved in the design of the protocol. Furthermore, the design may not allow for new innovations in techniques that could have saved significant time and increased patient recruitment and retention.
Typical examples:
- The protocol does not reflect the typical medical practice in the selected country
- Site set-up is delayed by contract negotiations with investigational sites
- Competitive studies change site selection
- Over-optimistic recruitment due to insufficient risk analysis
Inevitably, the trial runs late and the cost overruns become more significant. We seem to have forgotten the advice of Abraham Lincoln: ‘Give me six hours to chop down a tree and I will spend the first four sharpening the axe.’
value management
Many sectors facing the same challenge of getting the best value out of the supply chain have moved towards a value management approach. The UK office of Government Commerce has summarised a wide range of example case studies in which value management has saved significant project cost. For this approach an integrated feasibility team (from both the sponsor and preferred suppliers) focus on value not cost by:
- Understanding the challenge and the focus areas to really understand the objectives of the sponsor. This helps define the criteria for the success of the project
- Identifying alternative courses of action, including different techniques, approaches and methods that may be more cost-effective
- Analysing and evaluating different approaches against defined criteria to select the best option
The change, however, is not just procedural, it is also cultural with sponsors and suppliers adopting a more open approach to collaborative working. Some of the areas for change are:
- Joint leadership and commitment from the top, with a collaborative approach to project management. This eliminates the need to duplicate project managers in the sponsor and the CRO
- Rewards for organisations against balanced scorecard that measure the overall success of the study financially, in terms of quality, for the staff and the customers, not just the just cost
- An integrated knowledge management database using shared electronic data management systems
- A flexible workforce with strong team-working between the partners in the project
- Multi-disciplinary team working across all phases of the study
- Open book finance in which the true costs of the study are open and reward is based on a more holistic assessment of performance
Value studies are achieved via workshops
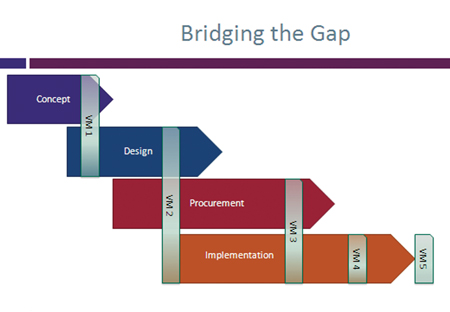
The processes for value management (VM) are well defined and include clear steps completed at each of the stages. A number of workshops are conducted at each stage of the project. The aim is to bring together the different teams in a collaborative view of the project’s objectives and strategy.
The main studies conducted are:
VM1: stakeholder needs, objectives and priorities
VM2: project definitions and options
VM3: value engineering to reduce cost
VM4: handover review
VM5: lessons learned
These workshops typically follow a three-stage structure:
- Diagnosis of issues with client and suppliers
- Workshop with the integrated team using VM techniques to generate solutions
- Follow up to ensure agreement of actions
In conclusion, value management is a tried and trusted way to reduce the overall cost of projects and has a successful track record in many sectors. It brings together the complete project team to understand the challenges and identify smarter, quicker and more cost-effective ways of completing the project, often by simple changes to the approach and requirements. The clinical research and development sector could learn much from these more collaborative and value focused approaches.
