The pharmaceutical industry has experienced unprecedented demand during 2020 during which huge efforts have been thrown into creating a COVID-19 vaccine as quickly as possible.
Taking a step back from these companies at the forefront of creating vaccines and new drugs, other factors — such as primary packaging — play a critical role in the global vaccine supply chain.
Glass offers better thermal stability for the purpose of sterilisation, enhanced transparency, superior impermeability, excellent barrier properties and chemical resistance, improved regulatory acceptance and lower environmental impact.
With vaccine trials showing hugely positive signs for completion in the near future, such as those from Pfizer and BioNTech, Moderna and AstraZeneca/Oxford University, the industry has recognised that there could be a shortage of vials to package the vaccine — particularly in the event that the vaccine requires several doses per person.
Estimates suggest that 15 billion doses would be required for a global vaccination campaign. Glass packaging manufacturers are now rising to the challenge of providing these much-needed containers.
Why moulded glass?
There are a range of glass primary packaging solutions to choose from, with pharma companies mostly making their decision based on criteria such as glass composition, surface treatment, processing and geometry.

Dr Jingwei Zhang
During the last few years, glass vial geometry and composition have been fine-tuned to meet the needs of pharma companies manufacturing biologics, complex drug products and vaccines.
Glass packaging can either be manufactured by injecting molten glass into a mould or by sectioning and finishing tubular glass. Each process and its final product have advantages and disadvantages.
Moulded processes offer greater design flexibility and the resulting end products have high mechanical resistance that reduces the risk of breakage.
Drug producers can therefore avoid issues with particles generated by glass fractures in a production line or, worse, inside a freeze dryer, avoiding loss of product or productivity during manufacturing.
This will be particularly vital in terms of manufacturing the COVID-19 vaccine; successful global immunisation will rely on rapid fill/finish and deployment without delays caused by breakages.
Glass packaging comes into direct contact with a parenteral formulation and can therefore impact product safety, quality and stability.
It is important that regulatory bodies control the contamination risk of aseptic fill/finish operations to ensure that glass packaging is as safe as possible for the patients using it.
Type I borosilicate glass is used worldwide for parenteral primary packaging; it’s a material with significant levels (~10%) of boron oxide, which is associated with inherently high hydrolytic resistance and a low coefficient of thermal expansion.
Hydrolytic resistance quantifies the extent to which glass is chemically inert and is measured in accordance with the methods defined in the European Pharmacopoeia (EP) 3.2.1 and United States Pharmacopoeia (USP) General Chapter <660>.1
These methods quantify (by titration) the amount of alkali released by a glass vial filled with ultra-pure water at elevated temperature(s). Low release rates are indicative of high chemical stability and a high level of hydrolytic resistance.
The low thermal coefficient of Type I glass enhances its suitability for thermal processes that enhance stability and shelf-life, such as pre- and post-filling sterilisation under relatively severe conditions (when required) and lyophilisation, an essential step for many biotherapeutics.
Chemical resistance is also superior in moulded products — a valuable characteristic for all formulations and often crucial for those with a high pH.
Table I shows the extractable profiles of Type I tubular and moulded glass vials (Type I composition, SGD Pharma) for silicon, sodium, potassium, calcium, aluminium, boron, barium and zinc, under different chemical conditions.
This demonstrates the enhanced chemical resistance of moulded glass vials. By contrast, tubular vials offer a high cosmetic aspect, which makes inspections more streamlined. Moulded glass vials are therefore an ideal complementary solution for pharma companies already using tubular vials.
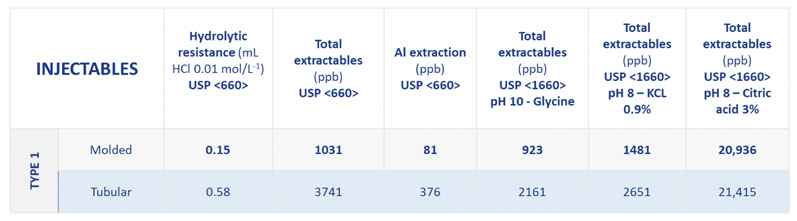
Table I: Extractables measured from Type I moulded and tubular glass vials (silicon, sodium, potassium, calcium, aluminium, boron, barium, zinc); *tests done on 10mL injectable vials for 1 cycle
As well as improved physicochemical properties, moulded glass offers enhanced production speed compared with tubular glass.
As well as increasing turnover, this confers a significant advantage for the rapid supply of vials globally in the case of urgent need: a single moulding line can produce more than 400,000 10 mL vials per day.
In addition, the traceability of moulded glass vials — effected by marks on the base indicating glass type, mould number and, often, production location — confers significant advantages.
Looking ahead
With COVID-19 presenting the industry with huge demands, the move to moulded glass highlights the importance of ensuring safety, sterilisation and compliance when providing such materials for vaccines. Patient health should continue to be at the forefront of the packaging industry.
Although tubular glass vials are helping the industry to meet the needs of global immunisation programmes, the advantages of moulded glass vials are now being leveraged in a move to secure the packaging supply chain for the upcoming COVID-19 vaccine.
As well as the beneficial physicochemical properties of moulded glass vials, which would be ideally suited to multi-use vaccine vials, the production process of moulded glass vials is around 10 times faster than that of tubular vials.
With Pfizer, Moderna and AstraZeneca all recently announcing that their COVID-19 vaccines are shown to be more than 70% effective in preliminary tests, it is likely that many of these vaccines will soon be available globally.
With this in mind and countries quickly ordering as many supplies as they can, the speed of vial production for pharmaceutical glass manufacturers and the ability of the moulded glass products to protect its contents, robustly, in transit, will be hugely advantageous.
Reference
1. USP <660>/European Pharmacopoeia (EP) 3.2.1, “Glass Containers for Pharmaceutical Use,” uspbpep.com/usp31/v31261/usp31nf26s1_c660.asp.
