Since that time, reports Tanya Tadey, Director of Laboratory Systems and Technology, Thermo Fisher Pharma Services, the list of identified N-nitrosamine impurities and impacted products has grown, led to recalls and, in the case of ranitidine, withdrawal from the market.3
The European Medicines Agency (EMA) and other regulators have responded by issuing requirements for marketing authorisation holders (MAHs) to assess and address the risk of N-nitrosamine impurities in their products.4,5
These requirements have presented companies with new and complex challenges when developing and validating analytical methods to detect nitrosamines in drug products.
Many pharmaceutical companies, manufacturing organisations and contract testing laboratories are looking to identify the most appropriate instruments that will support the risk assessments and routine testing required by regulators.
These instruments should be sufficiently sensitive and selective to unequivocally detect nitrosamines at levels specified by regulatory authorities. For example, the US Food and Drug Administration (FDA) recommends that products with daily doses of up to one gram are supported by methods with limits of quantitation (LOQ) at or below 30 ppb.
Even higher sensitivity may be required for high dose (>1 g) products or when multiple nitrosamines are expected to be present. Mass spectrometry (MS) and chromatography based methods are key technologies for N-nitrosamine analysis, as they provide the required sensitivity and resolving power to support different stages of analysis.

Choosing the most suitable instruments can be challenging for laboratories, however, as there is no one-size-fits-all approach to nitrosamine analysis and many factors need to be considered.
The multifaceted challenges associated with nitrosamine analysis
For organisations testing pharmaceutical products, the nitrosamine issue has generated many business-related and technical challenges.
Before deciding how to move forward on a technical and operational level, companies had to gain an in-depth understanding of the new requirements and resulting implications for testing. The EMA and FDA recognised the challenges faced by industry and, in response, held webinars and Q&A sessions. Despite this support, questions remain.
Regulatory agencies require laboratories to use validated methods to perform confirmatory testing of APIs and drug products whenever there is any risk for the presence of nitrosamine impurities.
Although laboratories are free to select which instruments they use to ensure they achieve compliance, many are unsure about which instruments to choose for the different types of analyses. Furthermore, laboratories must weigh up their options in the context of their own priorities, such as robustness or throughput.
General considerations for method development and validation
Pharma companies are under enormous pressure to implement instruments and develop processes to ensure compliance. The detection of N-nitrosamines in current products will ultimately lead to safer medicines; however, there is little room for error.
With huge repercussions for inaccurate N-nitrosamine analysis results, it is essential that results are consistent. False-positive results could lead to unnecessary financial losses resulting from product recalls, and the time spent on investigations and potential reformulation.
By contrast, the generation of false-negative results would mean that these potentially carcinogenic compounds remain present and undetected in pharmaceutical products.
When developing and validating methods for nitrosamine analysis, careful consideration of the product matrix, chemistry and potential sources of interference or contamination can help to avoid erroneous results. For example, nitrosamines may form during sample preparation if nitrosamine precursors, nitrites and secondary or tertiary amines are present together in the sample matrix.6
When these components are present during sample preparation — even at low levels — conditions known to accelerate nitrosamine formation, such as acidic pH and heat, should be avoided.

A widely cited example of in situ formation of nitrosamines owing to heat is NDMA formation during headspace gas chromatography (GC) analysis of ranitidine products.
Additionally, many plastic and rubber materials used during sample preparation and handling may contain traces of nitrosamines. By understanding the chemistry and potential sources of nitrosamine production, analysts can better adapt their methods to avoid erroneous results.
In addition to testing for nitrosamine impurities, laboratories may consider screening the drug product and ingredients for precursor chemicals, such as nitrites and amines that may contribute to the formation of nitrosamines.
By regularly monitoring precursors, laboratories can have data at hand to help identify and control potential sources of nitrosamine formation. If a nitrosamine appeared in the drug product downstream, for example, laboratories could inspect nitrite levels in excipients to identify any correlations.
Weighing up chromatography and MS technologies
When selecting specific instruments and technologies for nitrosamine analysis, laboratories will look to different sources for guidance, such as methods published by regulatory bodies detailing the determination of nitrosamines in specific drug products.7
For many, these published methods can serve as a starting point when looking to develop and validate methods for nitrosamine analysis. Even in these cases, however, the methods must be validated in-house to demonstrate suitability for the specific drug product being tested.
To date, methods have been published using a variety of platforms, such as LC-MS/MS, GC-MS and LC/high-resolution MS (HRMS). Each of these techniques has advantages depending on the specific nitrosamine and product being tested. Therefore, the application and type of testing (screening versus release testing) should be considered when selecting testing technologies.
Two commonly used systems for nitrosamine testing are HRMS and triple quadrupole MS (QqQ-MS). Having a high-resolution MS instrument is particularly beneficial for initial screening analysis to identify a new nitrosamine for which a standard may not be available.
It is also preferred for samples with complex matrices as the high resolving power can selectively detect the nitrosamine of interest — even in the presence of coeluting interferences. QqQ-MS, by contrast, may be the technology of choice for high-throughput analysis in a quality control environment owing to its ruggedness and ease of use.
However, more time and resources may be required to refine chromatography methods for lower resolution QqQ workflows compared with high-resolution MS, which is less reliant on chromatographic separation.
Both HRMS and QqQ-MS will need GC or liquid chromatography (LC) to separate the sample components prior to analysis. These highly orthogonal techniques can be applied during initial development and screening to ensure nitrosamines can be accurately quantified in the drug product matrix.
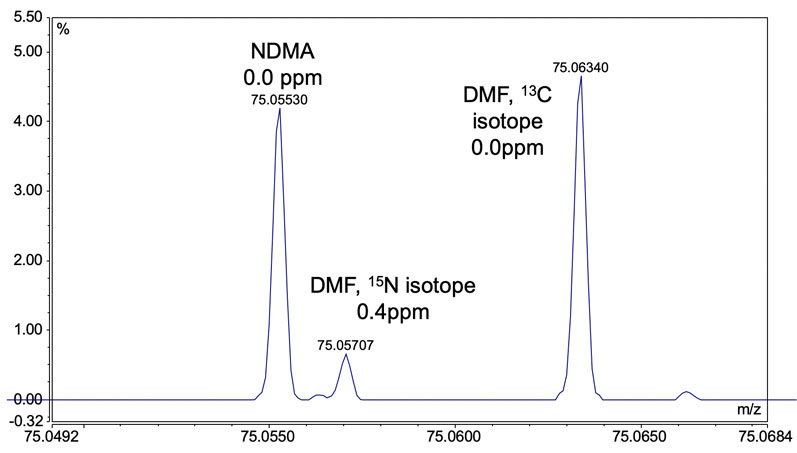
Figure 1: Mass spectrum of sample prepared from ranitidine drug tablet showing separation of NDMA and DMF; when an inadequate mass tolerance setting is used for data processing, DMF can cause an overestimation of NDMA
Compared with LC systems, an excellent level of sensitivity and chromatographic separation can usually be achieved with GC; however, it does require special considerations.
The heat generated during GC headspace sampling has the potential to generate nitrosamines in situ and must, therefore, be used with care. GC analysis also requires analytes to be volatile, yet sufficiently stable.
Achieving this for N-nitrosodiphenylamine, however, is only possible with time-consuming derivatisation steps.8 Ideally — and particularly in a high-throughput, quality control environment wherein simplicity is the goal — derivatisation would be avoided. Derivatisation lengthens the workflow time and is more complex for low-abundance impurities such as nitrosamines.
The importance of having sufficient mass resolution is demonstrated when considering two closely eluting compounds, such as NDMA and the residual solvent N,N-dimethylformamide (DMF) (Figure 1).
Generally, if these compounds cannot be resolved by high-performance LC, sufficient mass resolution to distinguish between the two will likely not be achieved with QqQ.
This potential pitfall was highlighted in a 2020 paper published by the FDA whereby a private testing laboratory overestimated the level of NDMA in metformin.8
The analytical technique used was high resolution time-of-flight mass spectrometry; however, with insufficient resolution, DMF interfered with the NDMA measurement. The false positive resulted in a costly product recall and could have been potentially avoided if a mass spectrometer with higher resolution had been used.
Rather than selecting one MS instrument for nitrosamine analysis, many laboratories may choose to have both QqQ and high-resolution MS instruments to support the complex requirements for nitrosamine testing.
A high-resolution MS instrument will be appealing for its high resolving power and selectivity, and typically provide more molecular and structural information than QqQ instruments. This can be particularly beneficial during method development and initial screening studies, or in the presence of complex matrices.
Alternatively, QqQ can also provide a level of sensitivity and quantitative accuracy that is well-suited to targeted analysis. Additionally, QqQ instruments are highly robust, which will be particularly valuable in routine testing environments.
Time to gain momentum in nitrosamine analysis
The EMA and other regulatory bodies have responded to the detection of nitrosamines in drug products by creating new requirements for pharmaceutical manufacturers.
Faced with these testing requirements, laboratories must now decide how they will implement analytical workflows to ensure compliance. As evidenced by previous reports of overestimated or even false positive results, there is a need for robust, sensitive and accurate instruments that support the analysis of nitrosamines in drug products.8
By carefully considering sample type, laboratory priorities and type of analysis, laboratories can find chromatographic and MS technologies that are best suited to their needs. Regardless of the instruments selected, a high level of product and chemistry understanding is essential to developing and validating an accurate and robust method.
Given the complex and high-stakes nature of nitrosamine analysis, laboratories may benefit from discussing their options with a vendor who can advise on how to take steps towards a workflow that will support nitrosamine analysis and their laboratory needs for years to come.
References
- www.fda.gov/news-events/press-announcements/fda-statement-fdas-ongoing-investigation-valsartan-impurities-and-recalls-and-update-fdas-current.
- https://doi.org/10.1177/2042098618823458.
- www.fda.gov/news-events/press-announcements/fda-requests-removal-all-ranitidine-products-zantac-market.
- www.fda.gov/media/141720/download.
- www.ema.europa.eu/en/human-regulatory/post-authorisation/referral-procedures/nitrosamine-impurities.
- www.ema.europa.eu/en/documents/referral/nitrosamines-emea-h-a53-1490-assessment-report_en.pdf.
- www.fda.gov/media/125478/download.
- https://doi.org/10.1208/s12248-020-00473-w.
