Nanoform’s new case study has shown that its CESS nanonisation technology enhances the pharmacokinetic properties of APIs and lowers the required dosage.
The Finnish company assessed the difference in suspension stability and pharmacokinetic behaviour between Dv50 350 nm piroxicam particles produced by its CESS process and Dv50 2 μm piroxicam particles produced by a standard micronisation.
Nanoformed Dv50 350 nm piroxicam particles and micronised Dv50 2 μm piroxicam particles produced suspensions that were easily re-dispersible.
Scanning Electron Microscope (SEM) images of the material show that the piroxicam nanoparticles remain as individual primary particles in suspension, and do not agglomerate.

Piroxicam nanoparticles showed improved in vitro dissolution compared to their micronised counterparts.
A further study compared the pharmacokinetic profile of Nanoformed piroxicam with the micronised piroxicam formulations. It demonstrated that Nanoformed piroxicam with 20 mg/kg oral dose has superior pharmacokinetic properties compared to those of the microparticle (p-value <0.01 at 80 mins) and standard particle reference formulation (p-value <0.01 at 80 mins), with faster Tmax, higher Cmax, and larger AUC.
No difference (>0,9999) was observed between the 5 mg/kg nanoformulated oral suspension and the 5 mg/kg nanoformulated capsule dosing groups, which indicates that both dosing formulations can be used in future studies.
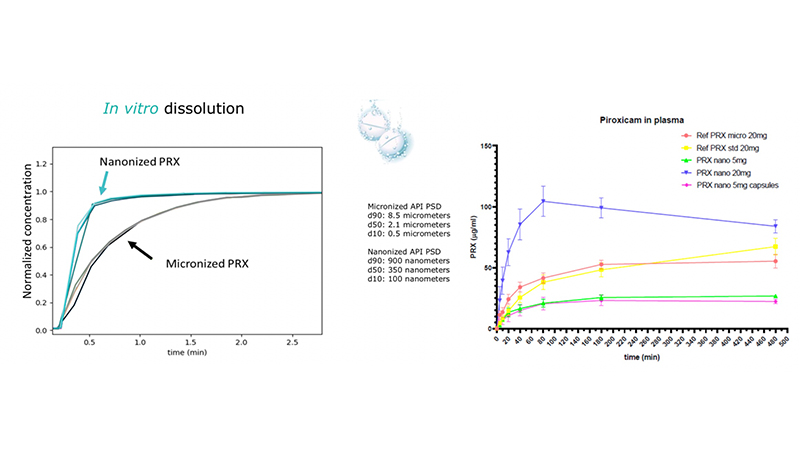
Based on these results, a possible dose reduction could be achieved with the 350 nm API particles. A larger dose reduction is expected when using smaller nanoparticles.
The results of this case study have important implications for the pharma industry. This is because decreasing the dosage of a drug is beneficial for the patient and because it can reduce manufacturing footprint, CAPEX, and environmental burden.
