Single-use filtration products were conventionally associated with small-scale processes in laboratories; however, biopharmaceutical manufacturers in particular have been keen to extend the advantages to commercial manufacturing with higher total process volumes. The challenge for process engineers is to optimise the separation techniques at their disposal in order to deliver the best technical performance (for example product yield) and acceptable economic performance, while complying with regulatory requirements imposed by the FDA, writes Lynne Deakin, Field Applications Specialist - Purification Division, 3M United Kingdom plc.
Today's disposable systems eliminate the majority of cleaning requirements, minimise the risk of product cross-contamination and bring further improvements in performance, making depth filtration an economic proposition for production environments – even in challenging processes like cell culture clarification.
Typical process
Cell cultures can contain bacterial, yeast, insect or mammalian cells, with mammalian cell culture production becoming the most widely used method. Manufacture of therapeutics derived from cell culture systems begins with fermentation of the appropriate genetically modified organism followed by purification of the cell-expressed therapeutic protein.
In harvesting the fermentation vessel, cell mass needs to be separated from the target protein. This is known as Upstream Processing (USP). Once sufficient cell debris has been removed from the culture fluid, purification continues by concentrating the harvest fluid into a manageable volume for chromatographic purification (removing soluble debris from the target protein solution) during Downstream Processing (DSP). In addition many elution buffers and regeneration chemicals applied to columns must also be filtered. When concentration and purification is complete, all that remains is to formulate and package the final product.
Depth filtration can be applied at many of these stages, and is especially suited to cell separation and clarification stages.
At the cell separation stage, getting depth filtration right reduces yield loss and minimises the problem of cell rupture (which can cause the release of proteases) whilst overcoming the general difficulties associated with filtration based on cell viability and cell titre. In addition, it is more effective than alternative separation techniques at removing cell debris, which can compromise downstream purification unit operations.
During the concentration steps, precipitates can form which must be removed by filtration; otherwise plugging of downstream chromatography columns can result, which is both a costly and time-consuming problem.
Suitability for scale-up
Scalability is a key issue as it has an impact on all these areas and depth filtration techniques have been proven to have inherent advantages over the alternatives. For example, tangential flow filtration (TFF) systems can also be evaluated at small scale and then used to specify production size systems. However, in order to obtain accurate sizing data with TFF systems, it is essential to use test filter devices with the same flow path length as are to be used in production system TFF filtration devices.
On the other hand, single-use depth filtration with identical flow paths and media in all devices, has demonstrated linear scaling from sub litre scale up to more than 10,000L when the same process fluids and process conditions are used.
Centrifuges pose a more difficult scaling issue. In order to obtain accurate scale up information at the clarification and purification stages, the same g-force and flow path device must be used. Mechanical constraints frequently make it impossible to scale centrifuge experiments. Scaling errors are inherent when batch centrifugation is utilised at small scale and continuous feed at large scale.
Depth filtration systems work on direct flow, so linear scalability is readily achieved by maintaining a constant flux rate (flow per unit area) and simply expanding the filter area in line with the batch volume. Where additional filtration capacity is required to accommodate a greater fermentation volume, modular systems provide a flexible solution and with single-use disposables there is no requirement to expand clean-in-place capacity.
In most cases the same pump package can be used, as relatively low pressures are needed to force fluid through the filter, which has an extremely low clean differential pressure. Unlike TFF systems where a high pressure differential is required and re-circulation pumps
TFF systems for cell separation are sized according to specific process requirements. If the harvest volume decreases or increases, changes in cross-flow re-circulation rate are necessary. If additional filtration area is required, it can be added in a modular fashion; however, re-circulation pump volumes must increase, often necessitating a new pump. TFF systems are also typically automated to control performance and any process changes require reprogramming. In some cases, increase in batch volume will involve changes to pipework and flow control sensors.
Centrifuge systems are also sized for specific process parameters. If the batch size increases, centrifuge capacity cannot be added. The only option is to purchase a new centrifuge. Despite these drawbacks, it is sometimes necessary to include centrifuges in the upstream process; for example when processing bacterial or yeast cultures where it is necessary to break open (homogenise) the cells in order to access the target therapeutic protein. This needs to be factored into the scaling equation.
To scale-up effectively, process development teams need to evaluate the filtration and purification process at laboratory R&D or pilot plant scales.
Fully encapsulated disposable filter capsules such as BioCap 25 Capsules and BioCap E0170, E0340 and E1020 Capsules from 3M provide the ideal media for scale-up evaluations at these volumes. Many pharma and biotech companies worldwide have scaled-up reliably and predictably to high area 12-inch and 16-inch diameter cartridge and encapsulated systems using small surface area bench top development kits that included BioCap disposable assemblies. For instance, studies by Singhvi et al. have confirmed that small scale data can accurately predict large- scale filter system requirements. Today, many laboratory investigations use micro-scale samples – some as small as 100ml – and in these cases scale-up measurements might be better left to the larger-volume pilot study, or at an intermediate stage if required earlier. For critical applications it is recommended that initial scaling data generated at bench scale is sanity checked at pilot scale.
Calculating production volumes
Volume, flow rate and pressure differential need to be measured in order to gain an accurate prediction of production-scale values. Measurements and calculations should not make assumptions such as proper performance of positive displacement pumps or calibrated flow meters - especially when processing high solid content solutions. Effective filtration areas rather than their nominal dimensions should be used for all the throughput and scale-up calculations. These figures should be available from the manufacturer.
Measurements should be repeated at least once to ensure reproducibility. This is especially important given the variation inherent in filtering biological products, so measurements are done in triplicate, to improve accuracy. A further complication is that filter performance is affected by processing variables further upstream. A representative sample of harvest or centrate material should be used.
Batch filtration operations are typically terminated at a predetermined pressure drop such as one- half of the filter's maximum rated pressure drop (for example, 26psid, or 1.8 barg, for Zeta Plus filter media from 3M. This figure is maintained across all scales and depth filtration is then scaled using a fixed flux value (fluid flow rate per unit of effective filtration area, in litres per minute per square metre). Once identified, this flux will be held constant irrespective of operating scale.
Calculating production values where time is not critical is therefore a matter of solving one of the two equations for A production.

Where V is the batch volume and A is the effective filtration area.
If time is a critical factor, the flux can be increased accordingly and the required filtration area A is calculated based upon the ability to process a batch of volume V at flux f in batch processing time P:
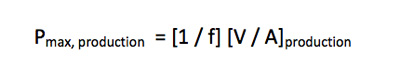
This equation is solved for A production, and the maximum batch processing time is used as a constant scaling factor throughout the experimentation:
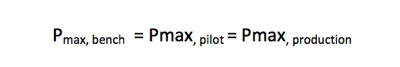
Therefore the following relationship should hold during scale-up and can be used to select batch volumes to be studied at each scale.
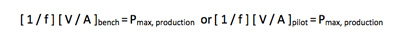
Filtrate quality
Determination of maximum reasonable flux, typically 2 - 10L/min/m², is based upon the filter's ability to deliver filtrates of minimum acceptable quality. So, in addition to measuring flow rates, volumes and pressures, fractions should be taken to determine the quality of the filtrate: yield of target protein and contaminants removed. This allows process engineers not only to predict the yield of the full scale process, but also to highlight issues that may be insignificant in the laboratory, but become problematic or costly in full scale production.
Examples include the presence of contaminants like endotoxins, host cell proteins (HCP) and host cell DNA (hcDNA). The presence of these negatively charged contaminants, if not removed during USP, requires costly chromatography stages in downstream purification. It is therefore important to remove as much as possible during the cell separation and clarification process.
There are a number of means to remove hcDNA including filtration and precipitation. Precipitation involves the addition of chelating agents which, although efficiently complex DNA resulting in precipitation, represents an additive to a process and can reduce yield of target protein which complexes with the hcDNA precipitate. Filtration, on the other hand, can remove DNA without the need to add additional chemicals to the process. Depth filter media has an inherent positive charge (Zeta potential) that attracts and binds negatively charged contaminants.
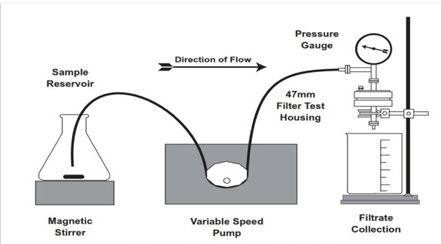
Figure 2. Zeta Plus filter media small-scale test apparatus
Consistency
Biochemical analysis during scaling trials can provide guidance for filtration and other separation processes at the production scale. A comprehensive set of scale-up measurements provides the basis for recommending the required filtration technologies (porosity, flow rates, differential pressures) and more importantly target protein yield.
How accurate this data will be depends crucially on ensuring that the filtration media is identical on devices of all sizes. First and foremost, this means selecting from a product range that fully spans the scale from lab to production. It also means choosing filter media that are guaranteed by the manufacturer to be consistent at all sizes, in terms of their pore structure, electrostatic and fluid flow characteristics.
Consistent performance from the bench to production is facilitated by scaling devices that aid media grade selection and filter sizing. Small scale devices in the Zeta Plus Encapsulated Filter System range from 3M, retain the lenticular filter design and vertical flow path characteristic of traditional cartridge depth filtration systems. Their use ensures consistency when migrating from cartridge systems to disposable capsule filtration as well as throughout the spectrum of single use systems utilised in Biomanufacturing from 0.5 L to 2500+ L Fermentation.
| Table 1: Zeta Plus Depth Filters, Tangential Flow Filtration (TFF) Systems and Centrifuge Systems Comparison | |||
| Issue | Zeta Plus Depth Filtration | TFF | Centrifuge |
|---|---|---|---|
| Scalability | Yes, Linear | Yes, Linear | Difficult |
| Effluent Quality | Excellent | May require additional filtration | Requires additional filtration |
| Yield | Excellent, > 95% | Good, may require diafiltration | Dependent on solids dryness achievable |
| System Flexibility | Easy to size up or down | Difficult to scale up | None - Fixed process design |
| Fixed Process Design | No | Yes | Yes |
| Shear Forces | Low | Moderate | High |
| CIP Validation | Simple - single use filters | Complex - Requires membrane re-use | Complex - May require equipment disassembly |
| Cross Batch Contamination | No - Single use filters | Yes - Membrane re-used | Yes - Difficult to CIP equipment |
| SIP Capability | Yes | No | Yes |
| Capital Cost | Low | High | High |
| Maintenance Cost | Low | Moderate | High |
| Consumables | Moderate - Filter replacement | Low/High - Dependent on membrane life | Moderate - Power consumption |
