Ensuring that this compound elicits the appropriate effect at the correct target site is of vital importance. Another regulatory requirement of drug development and manufacturing is guaranteeing that no inappropriate organisms are able to sustain themselves within the product or its packaging.
Antimicrobial preservatives are added to pharmaceuticals, medical devices, personal care products, cosmetics and food products to inhibit the growth of micro-organisms inadvertently introduced during the manufacturing process or during the use of products with multiple-dose containers.
Preservative efficacy testing (PET) — also known as antimicrobial effectiveness testing — is undertaken to ensure that the preservative maintains its ability to inhibit micro-organisms and is required by British Pharmacopoeia (BP), European Pharmacopoeia (Ph. Eur.) and United States Pharmacopoeia (USP) standards.1,2
This article will explain the importance of PET for manufacturers, providing examples of when specific challenges to preventing microbial growth might occur and how partnering with testing facilities such as Wickham Laboratories enables companies to comply with international regulations.
The process of PET
Limiting the growth of micro-organisms in various products helps to prevent them from spoilage and protects patients/users from possible infection. A number of factors determine the efficacy of a preservative, including the active component of the product, the formulation in which it is incorporated or the container/packaging in which the product is enclosed.
For example, a topical cream contained in a pot that the user dips their fingers into will be affected differently to a cream contained in a tube applied directly to the skin. If any of the components change, it is necessary to re-evaluate how the preservatives will continue to perform.
The PET method is relatively straightforward: efficacy is evaluated by challenging the preparation (in its final container whenever possible) with a suitable range of micro-organisms. Samples are taken at set time intervals and the surviving organisms are enumerated.
The preservative properties are adequate if there is no increase or there is a significant reduction in the number of organisms within a certain time frame. The specific criteria depend on the type of product.
Which organisms to use and when
The challenge organisms used are representative of a wide range of microbial types, encompassing both Gram negative and Gram positive bacteria, yeasts and moulds. BP/Ph. Eur. and USP regulations differ slightly in the test organisms required for certain pharmaceutical product preparations (see Table I).
PET should be conducted as soon as possible after the manufacture of a new product to ensure the preservative is compatible with the formulation.
A second challenge test is recommended after manufacture, often a few months later, to make sure the preservative has not broken down and lost effectiveness with time. It is also crucial to revisit PET whenever the formulation, manufacturing process or packaging of an existing product changes, as any of these can impact preservative performance.
PET is often done in dedicated testing laboratories, owing to their unique set-up and the manual requirements of the test. For example, Wickham Laboratories has the space required for large-scale PET, which involves numerous plates for multiple organism challenges across many time points.
PET can be conducted at the same time as a stability test, which provides the information necessary to demonstrate that a product’s efficacy lasts throughout the storage process. When samples are pooled at specific time points in a stability test, PET is done as well to track the action of the preservative during the course of the stability programme.
Some companies prefer their testing laboratory to use environmental isolates as the challenge organism. These isolates can be recovered from raw materials, finished products or production equipment surfaces. In this case, there are no pass/fail criteria as there would be with challenge organisms in the standard protocol, but the customer can gain insights into how their product might behave in the manufacturing suite during the 28-day standard.
BP/Ph. Eur. and USP methods require organism counts to be made at various time points up to 28 days, with the interval depending on the type of product preparation.
Method suitability
When the testing laboratory receives a new sample from a company for PET, method suitability/validation must be done before the test. The sample is purposefully inoculated with the challenge organism to check if there is a suitable recovery against a control.
If recovery is insufficient, PET cannot be completed because there is no way to tell if what has happened to the organism is derived from the action of the preservative.
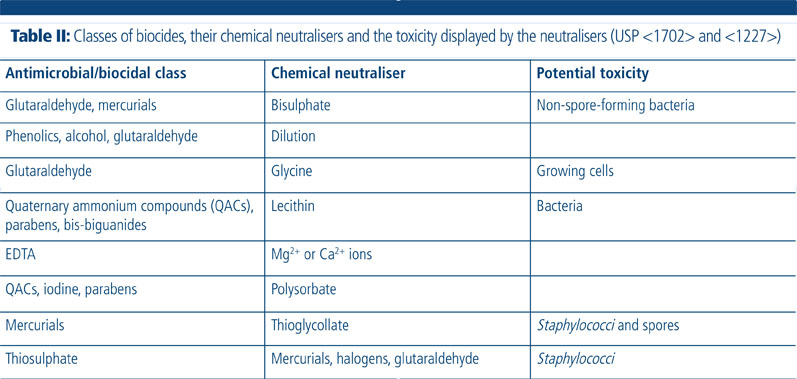
The neutralisation of the test solution’s antimicrobial properties with different neutralisers is required before estimating the number of viable micro-organisms; this ensures that the results are a true reflection of the number of viable micro-organisms present in the sample.
Validation can be done by chemical inhibition (preferred method), filtration and washing, dilution or a combination of these. The neutraliser must effectively inhibit the action of the antimicrobial without being toxic to the micro-organisms. The validation can be repeated using different neutralisers to accommodate for this potential toxicity (Table II).3 Method transfers from a previous laboratory may take place if it is still suitable for the product.
Challenges facing manufacturers
Certain factors can complicate the effectiveness of PET. Cough medicines in multidose containers are a good example of when patient compliance implicates the efficacy of the preservative.
The recommendation is to take the dose using a measuring spoon; however, many individuals may drink the medicine directly from the bottle, therefore potentially introducing unwanted micro-organisms to the product, which is likely to be used again. Manufacturers should take these factors into account when assessing the preservative component of a formulation and requesting PET.Cough medicines also provide an example of how changes in packaging require new PET. Recent developments in product packaging mean that some manufacturers have introduced child-friendly multidose syringes to administer the syrup, which plug into the top of the bottle, rather than relying on the syrup being poured into a spoon.
This change in packaging and method of dosing warrants a new PET, particularly because the syringe is reintroduced to the bottle after being in the individual’s mouth. The preservative requirements are likely to change.
Testing laboratories are faced with a wide range of products for PET. For example, even if there is no current standard for a product, facilities such as those at Wickham Laboratories are able to use prior experience to develop a new procedure to establish preservative efficacy.
Looking forward
Food, pharma and cosmetics companies are continuously revising and streamlining the ingredients in formulations for optimised efficacy and cost. Each time, preservative levels are likely to be re-evaluated and therefore require retesting for their ability to inhibit microbial growth in the product to a satisfactory level, according to regulation.
Although the British, European and US Pharmacopoeias set out clear requirements for the PET of pharmaceutical products, the regulations are less clear for other industries.
EU legislation pushed for more PET to be conducted in the cosmetics industry, outlined by the replacement of the Cosmetics Directive by the new EU Cosmetic Regulation in July 2013, showing that there are ongoing improvements to be made.4
By sending products for PET to experienced testing laboratories, manufacturers can be confident that almost any new product or formulation will be tested for preservative efficacy to make sure it is safe for consumers and meets regulatory standards. Research and development departments are constantly trying to optimise products and, as new innovations in formulations and packaging are developed, PET requirements will change and could become more complex.
References
- European Pharmacopoeia 7.0, Efficacy of Antimicrobial Preservation (5.1.3.): www.drugfuture.com/Pharmacopoeia/EP7/DATA/50103E.PDF (2011).
- United States Pharmacopoeia (USP 35), Chapter 51, Antimicrobial Effectiveness Testing: www.pharmacopeia.cn/v29240/usp29nf24s0_c51.html.
- S. Sutton, “The Antimicrobial Effectiveness Test,” www.microbiologynetwork.com/antimicrobial-efficacy-test.asp (The Microbiology Network, 2010).
- Official Journal of the European Union, Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products: https://ec.europa.eu/health/sites/health/files/endocrine_disruptors/docs/cosmetic_1223_2009_regulation_en.pdf.
