According to BCC research, global single-use technology in the biopharmaceutical market should reach a value of $5.9 billion by 2024, up from $3.5 billion in 2019, at a compound annual growth rate (CAGR) of 11.3% for the period of 2019 to 2024.1 Furthermore, Grand View Research has projected a CAGR of 12.9% from 2016 through to 2027.2
The primary drivers for this growth are improvements in cell culture processes, the expansion of the biotech industry and the increasing prevalence of personalised medicines.
Notably, single-use process skids are enabling innovative start-ups and contract manufacturing organisations (CMOs) to produce clinical trial material, monoclonal antibodies (mAbs) and cell and gene therapies at a greater pace.
The outcome is faster speed to market compared with reusable technology while ensuring consistent quality and safety. Here Mark Sitcoske, CEO and founder of High Purity New England, explores the single-use landscape and presents the benefits of single-use mixing technologies in a case study.
The benefits of single-use technology
CMOs have benefited greatly from single-use technology as one of its core use cases is to simplify multiproduct manufacturing implementation. This enables facilities to respond more quickly to changing supply demands and pivot when client needs change.
Other benefits include lower capital cost and greater flexibility in manufacturing. In addition, more efficient and faster turnaround times can be achieved for smaller batches as cleaning and sterilisation isn’t required prior to validation.
Businesses in the biotech space have shown particular interest in single-use. Many of the biotech market players are start-up virtual businesses with little or no manufacturing footprint — so they rely heavily on contract partners to create commercially viable solutions in their facilities.
The typical smaller footprint of single-use technology also allows sites to manufacture products in less space and offers the potential for the diversification of manufacturing processes at larger facilities.
Single-use mixers
When it comes to mixing technologies, biopharmaceutical manufacturing was revolutionised in with the introduction of single-use 2D and 3D process containers and filter assemblies for mixing and storage systems. Traditional stainless-steel mixers have moving parts inside (such as impellers) to mix.
Typically, they need a pump to add the fluid, a magnet to rotate the impeller to initiate mixing and then another pump to remove the media. Single-use technology often comprises plastic disposable components such as tubing and bags, offers biopharma companies more flexibility during manufacturing and eliminates the need to clean and reuse traditional stainless-steel equipment.
This helps to support pharmaceutical companies in their ongoing mission to improve product time-to-market.
Contamination risks are also significantly reduced as single-use technology components have been presterilised, often using gamma irradiation. Improved sterility can also reduce the risk of batch contamination. The cost benefits are two-fold.
First, single-use technologies require lower initial capex than stainless steel systems and can accelerate time-to-market; when combined, this delivers greater overall cost efficiency. Secondly, further cost savings can be realised through a reduction in ongoing operating costs such as cleaning chemicals and processes.
R&D departments are also realising the potential of single-use technology and this can create further benefits when potential drugs make their way through development and into the commercialisation stage. The potential to seamlessly scale-up by using single-use equipment and strategies can lead to significant reductions in process rework and inefficiencies that are typically expected in less flexible processes.
Case study: improving process and reducing contamination risk
LFB, a French biopharmaceutical company, conducted a study to understand the potential benefits of using the ClearMixx (QuattroMix) single-use mixing system. This study was the result of a collaboration between High Purity New England (HPNE) and LFB-USA to evaluate the use of this technology as a replacement for the existing ultrafiltration/diafiltration (UF/DF) system’s stainless steel retentate vessel in the LFB-USA process.
The acceptance criteria for the study were based on the performance of the current validated endpoint buffer preparation process parameters, which achieve a conductivity of 3 ± 1 mS/cm at 6 diavolumes (DV). Real-time samples were taken for conductivity measurements on three salt runs and one mock buffer preparation run using the actual buffer system.
For the final mock run, pH and conductivity were also taken after the final DV (in accordance with the approved batch record). The generated data was evaluated with results showing an acceptable conductivity level and confirming that the ClearMixx (QuattroMix) system is a suitable replacement for the stainless steel UF/DF feed tank.
Description
The existing UF/DF process begins with the first diafiltration: the elution buffer is added to the concentrated eluate for two DVs, adding in buffer and taking the water out to get rid of any contaminants that might be in the fluid while maintaining a specified weight.
During the second diafiltration, the elution buffer is exchanged for the final formulation buffer. During these steps, a homogenous mixture must be maintained to complete an efficient buffer exchange. Homogenous mixing was achieved via the overhead mixer in the UF/DF feed tank, which is verified in real-time using a standardised tachometer.
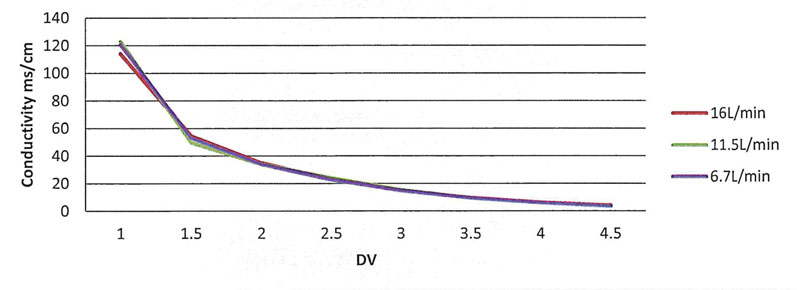
Figure 2: Conductivity (mS/cm) for each 1.5M NaCl solution run
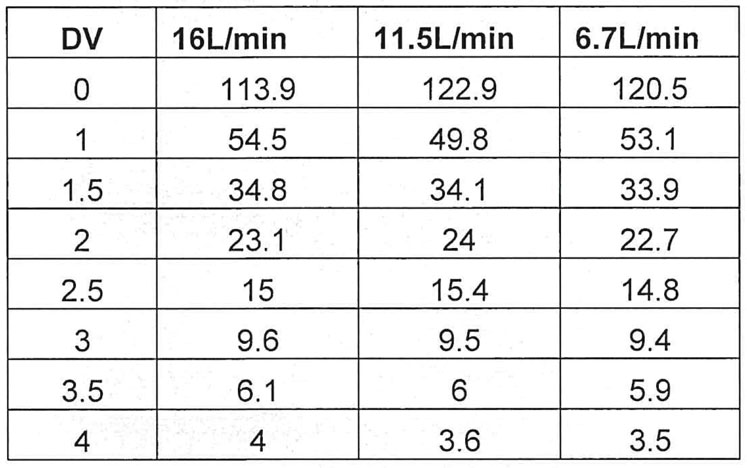
Table I: Conductivity (mS/cm) data for 1.5M NaCl solution runs based on flow rate
The next step is concentrating the chromatography eluate down to –30 kg within the UF/DF stainless steel feed tank. Conductivity samples are taken and tested in real-time during the second diafiltration. A final conductivity and pH sample are taken post DV2 to ensure that the diafiltrate is within the final designated specifications.
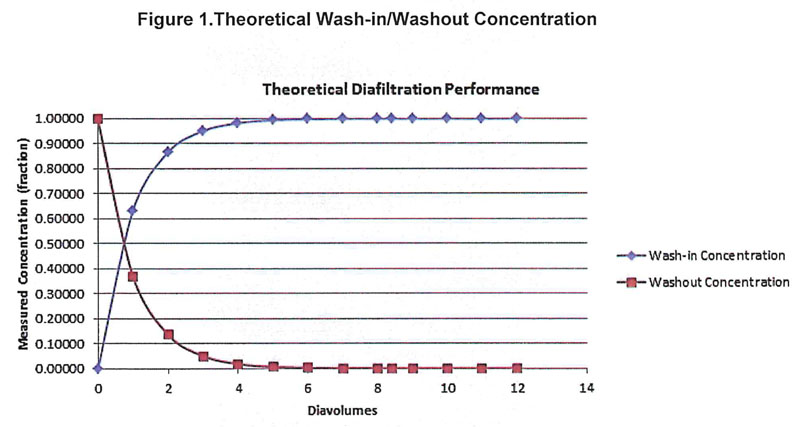
A theoretical curve was created to show the expected wash-in/washout concentrations of the solutions per diavolume (Figure 1). It is expected that after 6 DVs, the initial solution will have been sufficiently replaced. Based on the performance of the validated process, the acceptance criteria for the study is that the conductivity must be 3 ± 1 mS/cm after 6 DVs.
This measurement is in accordance with the conductivity critical process parameter (CPP) found in the batch record.
The feasibility study was set up in two stages. First, three diafiltration runs, using water and 1.5M sodium chloride (NaCl) were performed followed by one mock diafiltration volume using the actual elution and final formulation buffers. The initial three runs were executed at the chosen flow rates of 6.7 L/min, 11.5 L/min and 16L/min.
The flow rates were calculated by first measuring the retentate permeate flow rates volumetrically then subtracting the permeate flow rate from the retentate flow rate. This was done for all three flow rates prior to execution of the protocol.
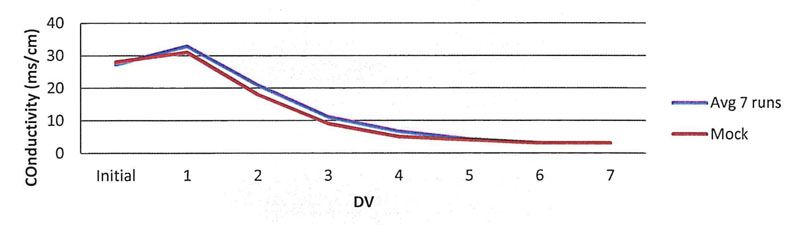
Figure 3: Comparison of the conductivity recorded during validated runs compared with the mock run
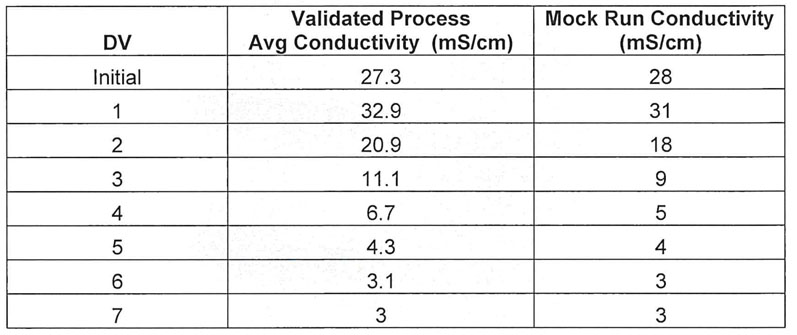
Table II: Average conductivity values of the validated process versus the mock run
Permeate flow rates were confirmed volumetrically at the beginning of each run, and throughout each run, through the permeate sample line. For all runs, the retentate weight was maintained between 33.0 and 31.0 kg to represent worst-case scenario conditions.
Results
The data produced and reviewed from the executed protocol provides evidence that the HPNE QuattroMix single-use mixing system can provide efficient mixing of the elution and final formulation buffers during diafiltration. The initial runs using the 1.5M NaCl solution showed that the conductivity values for each flow rate met the 3 ± 1 mS/cm in 6 DVs specification (Table I, Figure 2).
The conductivity curve produced from the final mock run is similar to the observed results in the validated process (Table II, Figure 3).
The tubing was changed to 73 silicone tubing prior to the execution of the final mock run. At the start of the mock run, the buffer exchange was visibly ineffective at creating one homogenous mixture. The flow rate of the pump was increased to 11.5 L/min and the run was completed at that flow rate.
Conductivity samples were taken from both the bottom and top of the bag to confirm homogeneity. The top and bottom sample conductivities were similar, indicating a homogenous mixture (Table III, Figure 4).
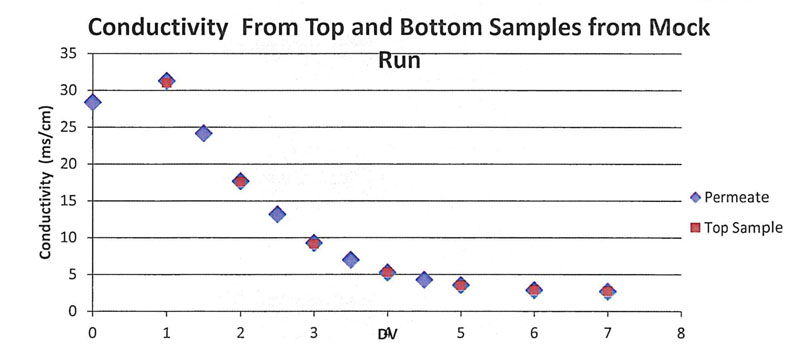
Figure 4
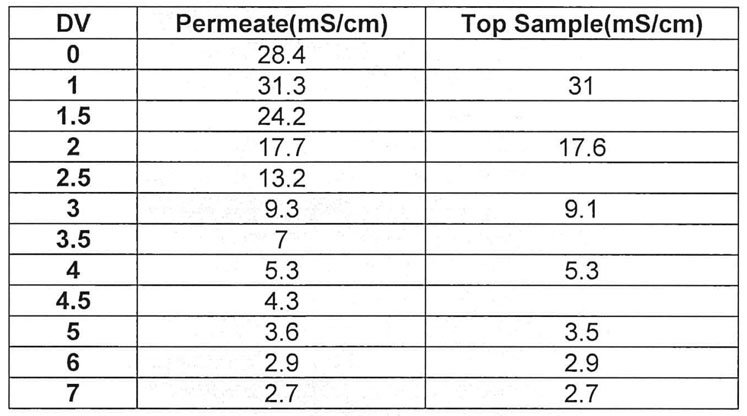
Table III: Conductivity of top and bottom samples per diavolume from the mock run
Results
The collective data indicated that the HPNE ClearMixx (QuattroMix) single-use mixing system can efficiently replace the stainless steel UF/DF feed tank. The change to a disposable mixing system creates a process improvement that reduces the risk of potential product contamination and lowers cost-expenditure by eliminating the reuse and cleaning of process equipment.
Conclusion
As the drug development pipeline increasingly focuses on personalised therapies that inherently require more agile, multiproduct manufacturing capabilities, more companies are set to adopt single-use technology. These well-engineered solutions are proven to reduce time, costs and cross-contamination risk during both downstream and upstream manufacturing.
References
- www.bccresearch.com/market-research/biotechnology/single-use-technologies-biopharmaceuticals-report.html.
- www.grandviewresearch.com/industry-analysis/single-use-bioprocessing-market.
