In the pharmaceutical industry, glass is by far the dominant material used to package liquid and lyophilised products. It offers a number of benefits, including its impermeability and chemical inertness for drug product stability, transparency for ease of inspection, thermal stability for flexible use and processing, low extractable and leachable characteristics, and cost.
Although glass breakage events occur at every pharmaceutical company, the methods of strength or reliability testing and fracture analysis (fractography) remain relatively unknown — and severely underutilised — to determine the root cause of failure and make the necessary changes to reduce future occurrences.
Fractography is the science of analysing the macroscopic and microscopic fracture patterns of cracked or broken objects to qualitatively and semi-quantitatively determine the root cause of failure.
Importance of appropriate strength testing strategies
Owing to the high complexities and the low overall incident rate of glass breakage, the strategy of today’s pharmaceutical industry is to forgo strength testing and assess the criticality of surface flaws/non-conformities by using optical/visual inspection with defect manuals. The danger involved with this approach is that defect manuals are designed for the cosmetic assessment of containers and the categorisation of non-conformities (disturbances) according to their lateral dimensions.
They cannot provide a criticality assessment with respect to container strength, because optical inspection is unable to deliver the information required to assess the criticality of a disturbance in terms of strength.
So, judging the criticality of disturbances in terms of strength, solely based on optical appearance, can lead to misinterpretation. Implementing appropriate strength testing strategies into production processes can help to lower the risk.
Fractographic investigations: three examples
Example one: SCHOTT pharma services was contracted by a client who was filling a clinical trial product into vials and, during post-fill inspection, found that approximately 20% of the batch had chatter marks or “scuffs” of varying size that were detected by visual inspection after processing. The pharmaceutical company was concerned about the risk of breakage at the clinic and contracted strength testing to be done to assess the strength criticality of the scuffs — and whether or not the scuffed containers could be safely used.
Burst pressure testing was done on 100 samples featuring scuffs, 92 samples without scuffs and 43 control samples (vials that were processed but removed after depyrogenisation). Data evaluation revealed quite similar distributions, with the vials featuring scuffs having slightly higher strengths than the vials without scuffs. This difference in strength was mathematically determined to be statistically significant.
Additionally, a fractographic examination of every broken sample from the rejected lot showed that not a single fracture origin coincided with a scuff. This means that although scuffs indeed represent a cosmetic defect, in this particular case they were proven not to decrease the container strength compared with containers without scuffs.
Example two: Strength testing is also invaluable for determining or selecting appropriate containers and/or drive springs for auto-injection devices. SCHOTT pharma services was contracted to perform flange strength testing on glass syringes by a client who wanted to determine and compare the device failure probability when using two different drive springs on three different lots of glass syringes.
While performing flange strength experiments with a slow, constant load rate as a destructive lifetime test, all syringes exhibited breakage in a range of forces far above the range of the application forces. To get a better understanding of failure probability under real conditions, a suitable continuous statistical distribution function was fitted to the strength data as a first step.
In a second step, the mechanical loads of the true force-time profiles of the two drive springs were transformed to single “equivalent” force values, which can be compared with the strength experiment data. An estimation of the failure probability of the syringes under the load of the two different drive springs is then achieved by extrapolating the fitted continuous statistical distribution function to the two equivalent force values of the drive springs.
Example three: A last example demonstrates the effectiveness of root cause analysis and corrective action when combining strength testing with systematic fractographic analysis. SCHOTT pharma services was contracted to perform syringe strength testing on samples before and after a particular processing step that was sporadically introducing observed surface flaws.
The client wanted to know the extent of strength decrease and whether or not the observed defects were responsible for the strength decrease. Burst-pressure testing of the syringe was conducted on samples drawn before and after the suspicious process step, showing a statistically significant decrease in strength in the samples after the processing step, as well as a narrowing of the strength distribution.
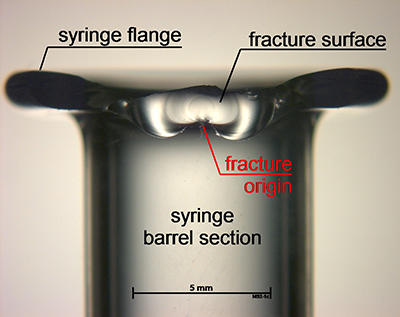
Figure 1: Post-processing fracture origins were detected in the flange region
Assessment of each syringe after breakage to determine the location of the fracture origin found an enlarged population of fracture origins in the flange region after the processing step (Figure 1), as well as a second large population in the shoulder region in the lower strength regime — as was found in the samples before the step (Figure 2). So, the combination of systematic strength testing with fractographic examination was able to reveal damage mechanisms on the outer surface in the flange and the shoulder section of the syringes.
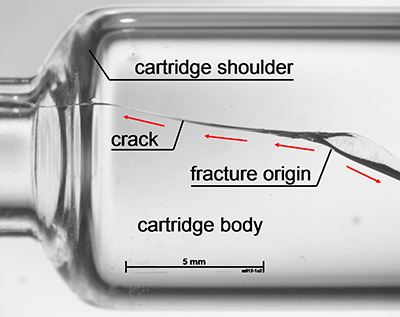
Figure 2: Fracture origins in the shoulder region of syringe barrels
With this type of insight, appropriate corrective and preventive actions were suggested to eliminate the damage mechanisms and thus successively improve the strength of the product.
Conclusion
Strength testing methods, combined with fractographic investigations and appropriate evaluation procedures, are available to provide the pharmaceutical industry with methodologies to determine the root cause of glass breakage events during processing, filling, shipping or during administration, and help to identify effective corrective actions.

