The development of innovative biopharmaceuticals to address unmet medical needs becomes increasingly important in the competitive market for biopharmaceuticals and is accompanied by evolving regulatory expectations. Close assessment of critical elements of a given protein often confronts developers with challenging pharmaceutical properties with regard to demands placed on the product’s stability, to the manufacturing process, and to preclinical or clinical expectations. The quality of the biopharmaceutical product and its readout by biophysical and biochemical analytical methods, therefore, is an essential part of the development process.
These analytical methods are involved in all stages of the development, serving as the fundamentals for process development and manufacturing. They include a broad spectrum of techniques from standard methods as described by European and United States pharmacopeia to very specific methods for detailed characterisation, which are designed to monitor properties of the specific biopharmaceutical, for example high resolution mass spectrometric analysis or in-vivo analysis of degradation pathways.
Key element API
Determining the protein concentration of the active pharmaceutical ingredient (API) is one of the key elements present in the specifications of each medicinal product recommended by all regulatory bodies and described in ICH guideline 6B (1).The importance of the quantitative determination is reflected by the fact that dosing of patients is directly related to the protein concentration of the drug substance (DS) and drug product (DP) of the medicinal product. Hence, the requirements for the protein content determination method are very strict and there is an eminent need for high precision and accuracy. To meet both criteria is highly complex and requires a sophisticated development strategy as pointed out in Protagen’s whitepaper “Development of an accurate, precise and robust mothod for determination of the protein content of biopharmaceutical therapeutics”.
Accuracy meets robustness
Biopharmaceuticals often show a direct correlation between dose and corresponding activity. Variability of the dose could therefore lead to a pharmacokinetic profile outside the intended activity range. Additionally, the determined protein content directly impacts several other assays, such as potency and bioassays.
The given unique molecular properties of the protein of interest determine the development strategy and followed protein content method. The target concentration range for the manufacturing process as well as the viscosity of the solution, which is mainly determined by the API concentration and the selected formulation excipients are key factors, which need to be considered during the development of the procedure as well as for qualification and validation. However, not only meeting the high standards for precision and accuracy, but also the robustness of the method, may have a significant impact on the workload, timeline, and costs of the development phases.
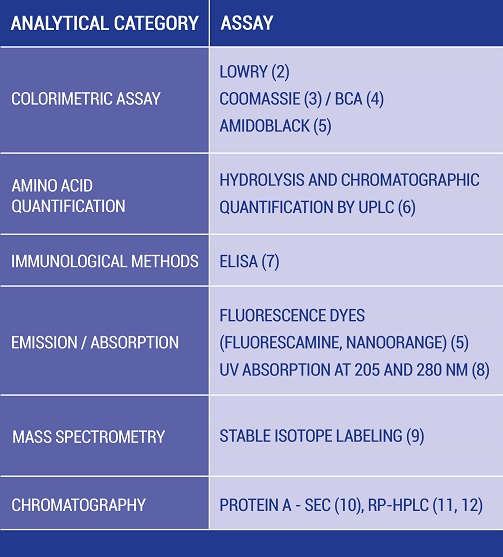
Table 1: Overview of protein content determination methods
Risk management
The corresponding risks are often underestimated by early phase development stakeholders, but can well be mitigated by thorough method development, qualification, validation, and life-cycle management. The protein concentration, which is determined throughout the entire process and all phases of biopharmaceutical production, will be used by many different laboratories for a variety of intended purposes. As such, the method must not only be highly robust but also method transfer must be straightforward.
Table 1 gives an overview of analytical methods for protein content determination: Several methods can be used for determining protein concentration during biopharmaceutical development.
Each analytical method shown in Table 1 comprises it specific benefits and pitfalls and the selection is often depending on the application and purpose. One of the commonly applied techniques is the protein content determination by UV absorbance.
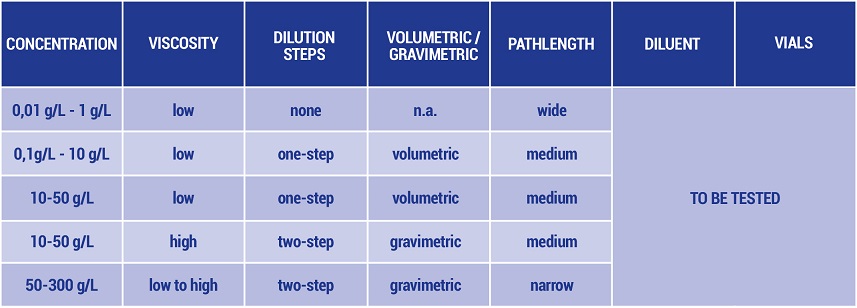
Table 2: Recommended UV methods suitable for different expected concentrations
In Table 2 a matrix is shown that supports the selection and development of a precise, robust protein concentration method by UV absorbance: For high concentrations of monoclonal antibodies, a one- or two-step gravimetric dilution in combination with a medium or narrow pathlength represents a promising starting point for method development.
More information on this topic is available in Protagen’s recent whitepaper: Download here
