The increasing dominance of biologics in the pharmaceutical pipeline, the more rapid market entry of biosimilars and the pressure to bring new products to market faster — at lower costs — are all having a knock-on effect on fill-and-finish operations. As new drugs are commercialised, manufacturing is both scaled-up and scaled-out, with production taking place in parallel across a number of facilities to supply local markets.
This strategy is an important way to derisk the supply chain by reducing reliance on a single, centrally located production line and improving agility by manufacturing product in various geographical areas to overcome potential shortages in certain regions at times of high demand. It also facilitates the flexibility offered by partnering with CMOs and CDMOs.
Key to this strategy, however, is a standardised machine concept that can operate with ready-to-use (RTU) packaging components, such as the SCHOTT iQ® platform, which allows glass syringes, vials and cartridges of different sizes and configurations to be filled on a single machine. Frequent changeovers between batches result in dedicated filling lines often being left idle as demand changes. This can seriously reduce the overall equipment effectiveness (OEE), which is a product of line availability, performance and uptime. Novel flexible filling lines can operate at much higher OEE rates owing to the lack of lengthy interruptions and downtime, which translates into time and money saved.
SCHOTT iQ® components are manufactured in state-of-the-art clean environments using high-quality materials
Although new container options are entering the market to meet the increased need for flexibility, to assess the economic feasibility of a switch from traditional to RTU containers requires a thorough and holistic understanding of all options and parameters. Deciding on a fill-and-finish concept that needs to be economically beneficial for several years, or even decades, means that all costs must be carefully balanced against each other.
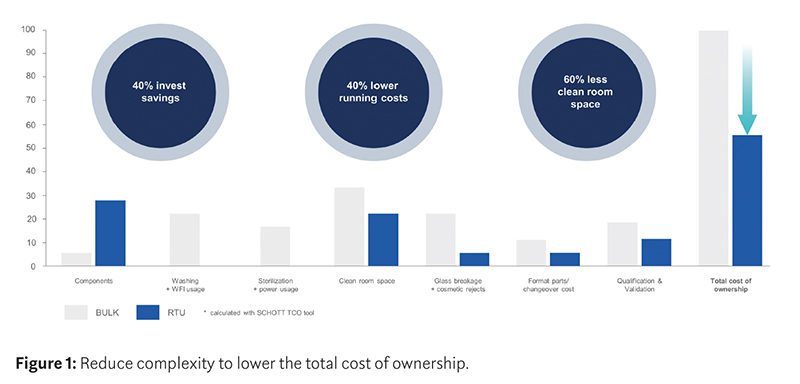
Economic viability is best assessed by looking at the total cost of ownership (TCO). This includes initial capital expenditure as well as recurring costs throughout the whole project lifetime and offers a clear picture of the machine and container combination that facilitates operations. A number of factors should be taken into consideration, including the location of the facility, the duration of the project, the types and number of different containers to be filled, the filling machinery to be used and the batch size (Figure 1).
When assessing the choice between traditional containers and ready-to-use systems, the implications go far beyond the simple cost of the components. Although RTU alternatives are more expensive than bulk containers, when processed on flexible filling lines they can offer significant cost savings and — owing to the quality of the containers — also enhance patient safety and regulatory compliance.
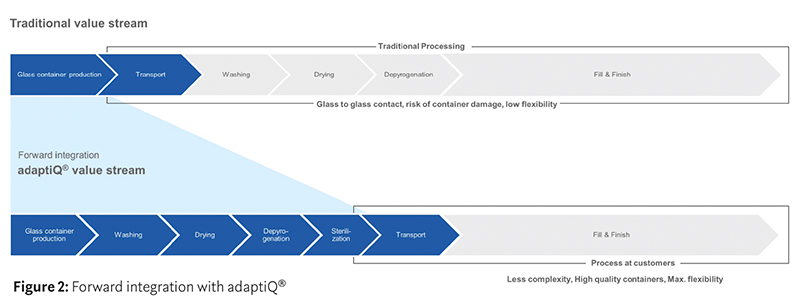
In the traditional value stream, glass containers are produced and transported to the pharmaceutical manufacturer where they are fed into the filling line, washed, dried and depyrogenated ready for the fill-and-finish process. As all container types have different dimensions, separate filling lines in individual cleanrooms are required for vials, prefilled syringes (PFSs) and cartridges.
With RTU systems, such as the SCHOTT iQ® platform, the value stream is forward integrated to reduce complexity and maximise flexibility. All the containers fed into the filling line have already gone through a standardised, validated and cost-optimised washing and sterilisation procedure. SCHOTT iQ® is built on compliance with all relevant industry standards; because the different container formats can be filled on a single filling line, there is a significant reduction in the initial investment in machinery and cleanroom capacity as there is no requirement for washing or depyrogenation stations (Figure 2).
SCHOTT Pharma uses trusted partners to do efficient and highly standardised ETO sterilisation procedures. Sterilisation cycles are optimised to have minimal environmental impact (A rating) and residuals in the final RTU container are below detection limit; thus, they’re significantly lower than industry wide accepted ISO standards.
SCHOTT iQ® components are manufactured and processed in state-of-the-art clean environments using scientifically selected high-quality materials. Furthermore, individually nested configurations have been designed to avoid glass-to-glass contact, thereby reducing scratches or microcracks. The absence of glass-to-glass contact results in fewer rejections by reducing the risk of particle generation, preventing cosmetic defects and decreasing breakages on the filling line. This ultimately reduces both costs and wastage.

Transportation to the plant and handling of the containers at the fill-and-finish facility have the potential to generate particle contamination. RTU systems offer a major mitigation of the risk. For instance, the design and material of the RTU packaging provides sufficient protection against mechanical damage, such as bumps and knocks, during transport and handling. Furthermore, validated secondary packaging not only preserves the aesthetic quality of the containers but also prevents tears in the material that would breach the sterile barrier on the tub and Tyvek® level. By using the secondary packaging to transport the containers in the fill-and-finish line (nested filling), the high cosmetic quality of the containers is maintained throughout the whole process as glass-to-glass contact is eliminated (Figure 3). This is in stark contrast to traditional filling lines in which the containers bump against each other and induce a significant number of surface defects.
The nest-and-tub concept was first developed for RTU prefillable syringes and has been the industry standard for approximately 30 years. Based on this established concept, SCHOTT Pharma offers a wide range of containment solutions that sit within harmonised and standardised secondary packaging. All materials required for secure RTU container storage and sterilisation are carefully selected and thoroughly investigated. The aim is to select materials that are unlikely to create particles and will protect containers from external sources of particles and contamination, as well as enabling efficient processability on customer lines.
The true key to leveraging the advantages of an RTU system is standardisation
Besides the capital investment required, it is also important to consider the ongoing running costs of the fill-and-finish operation. For example, the number of change parts needed on the filling line when bulk containers are used is far greater than for an RTU system. Furthermore, as the washing and sterilisation of RTU containers has already been done prior to their arrival at the facility, significant savings can be realised by eliminating the need for WFI loops and the expensive energy costs of heat tunnels. The high level of automation on the RTU lines also means that fewer operators are needed, thus also reducing labour costs and decreasing the risks associated with human intervention.
Another area in which an RTU platform has major advantages compared with bulk container filling is qualification and validation. On a traditional line, significant resources and time are required, particularly if the different containers are provided in bespoke packaging, meaning that the qualification steps are multiplied. However, because the total number of process steps is reduced for RTU containers, the qualification effort can focus on the aseptic filling and closure steps. A standardised platform for all containers unifies the transfer steps to get the containers into the aseptic core (debagging, decontamination, delidding) and these only need to be qualified once. This can ultimately speed up the installation of new filling lines and reduce valuable time to market.
Pursuing a single standard method of filling allows manufacturers to maximise the utilisation of each filling line. So-called multiplexing or modular manufacturing turns the traditional model on its head. Rather than custom designing a filling line around a specific drug many years prior to the launch of the product, a manufacturer can install filling capacity first, using modules equipped to fill a number of different RTU containers, then decide on what to fill and shift capacity and even the filling location as needed. Such flexible filling lines can process different RTU containers and closures on the same line, allowing batch size variations to meet changing market demand or manage a multiproduct portfolio.
The SCHOTT iQ® platform can be used with more than 50 machine types from a broad range of global vendors. To increase machine compatibility, the approach developed by SCHOTT Pharma uses a standardised 3-inch tub for all sterile glass primary packaging containers. This approach is now followed by all leading industry providers, thus enabling pharmaceutical companies to purchase second-source packaging without complex changeovers on their flexible fill-and-finish lines.
The true key to leveraging the advantages of RTU systems is standardisation. It enables flexibility while simplifying and speeding up processes … and saving costs. Implementation, validation and ramping up new filling lines can be done in shorter time frames while enhancing the quality of the containers. The SCHOTT iQ® platform product range is available globally. The online shop and stocks of fast-track items for quick shipping offer immediate supplies of small quantities direct to the customer’s door, enabling pharma companies to focus on developing best-in-class drugs and worry less about packaging components.

