Prefilled syringes (PFSs), such as SCHOTT TOPPAC infuse, is one possible solution; they offer a number of advantages to hospitals and critical care facilities, including fewer medication errors, improved patient safety and the option to reduce the hospital’s total costs.
Most injectable drugs administered at hospitals are infused intravenously as this allows a fast onset of action, a high degree of accuracy and nearly 100% bioavailability of the medication.1 However, the preparation and administration of these drugs is a complex and time-consuming process, particularly when the conventional use of a vial, ampoule or bag with a disposable syringe is concerned.
Prefilled syringes are suitable packaging solutions for IV drugs and have a wide range of advantages in the challenging hospital environment, including the faster preparation and administration of drugs, decreased waste, a lower chance of microbial contamination and a reduced risk of medication errors.2
When evaluating the key features of an optimal PFS for IV drugs, several factors should be considered, including compatibility with key hospital devices, such as needleless connectors, and the syringe barrel material. The barrel material is a crucial component of a PFS as it has a significant and direct influence on the stability and shelf-life of the filled drug. There are three common material options for PFSs barrels:
- glass: borosilicate glass
- standard polymers: polypropylene (PP)
- high-performance polymers: cyclic olefin copolymer (COC), cyclic olefin polymer (COP).
Borosilicate glass is a specialty glass developed by Otto Schott in 1911 and used since then as the benchmark material in primary pharmaceutical packaging for ampoules, cartridges, vials and PFSs.3 It shows a high level of chemical durability, has excellent barrier properties, is inert and has a low extractables and leachables profile.
These properties make it an ideal primary packaging material option for many parenteral drugs, such as vaccines and biologics.

© SCHOTT AG
For IV drugs, however, additional container-specific properties need to be fulfilled, such as the ability to produce large syringe formats and compatibility with needleless connectors. The production of borosilicate glass tubing and the subsequent conversion into a syringe are complex production processes that involve high temperatures and require significant manufacturing expertise.
With increasing size, the tight tolerances and precise container syringe geometries are getting close to the limits of technical and economic feasibility.
Furthermore, glass has a higher propensity to break, which may lead to a malfunction of the IV syringe and, ultimately, to a delay in drug administration, especially when combined with needleless connectors. Reported adverse events of IV glass syringes include syringe tip breaking, needles detaching during injection and syringes breaking or jamming the IV lines or other patient use lines.4
For these reasons, alternative materials such as polymers are gaining more importance as a solution.
According to a survey by SCHOTT AG, healthcare professionals seem to prefer polymer syringes as well. A poll of 660 healthcare workers in the US and six European countries revealed that 77% prefer a polymer syringe to a glass one.
Polypropylene (PP) is the standard material for disposable syringes in hospitals. PP is a cost-effective polymer material that allows for the easy production of a wide range of syringe formats, from small volumes of 1 mL up to larger formats of 50 mL or even more. Medical-grade PP has a high level of purity and is used to make disposable syringes for hospital use or for PFSs to store saline for flush syringes (to clear intravenous lines, for example).
However, when used as a container material for drugs, PP should be treated with caution. PP has low oxygen and moisture barrier properties and PP syringes often have a high level of extractables and leachables. These properties can directly affect the stability and shelf-life of the filled drug.
For example, Lorazepam has a stability of 48 h, Sufentanil has been shown to be stable for 28 days and others, such as Fentanyl, can stay stable for up to 100 days under optimum storage conditions.5–7 For these reasons, the US FDA has cleared PP syringes as medical devices for general purpose fluid aspiration and injection only, but not for use as a closed container storage system for drug products.8
PP also has a lower transparency than glass and COCs. This can pose a challenge to automatic inspection after filling and can hamper the final assessment of the drug by the healthcare professional prior to injection. COCs and COPs comprise a relatively new class of high-performance thermoplastics that are characterised by high purity and transparency. The two main types of material in this class differ in terms of the production process.9
COCs combine the advantages of borosilicate glass and standard polymers: they are chemically inert, have good barrier properties, low extractables and leachables, and are commercially available in large formats up to 50 mL. As they are a relatively new material for PFSs, production capacity is limited at present. However, manufacturers are currently ramping up global production so that this issue will become less critical in the future.10
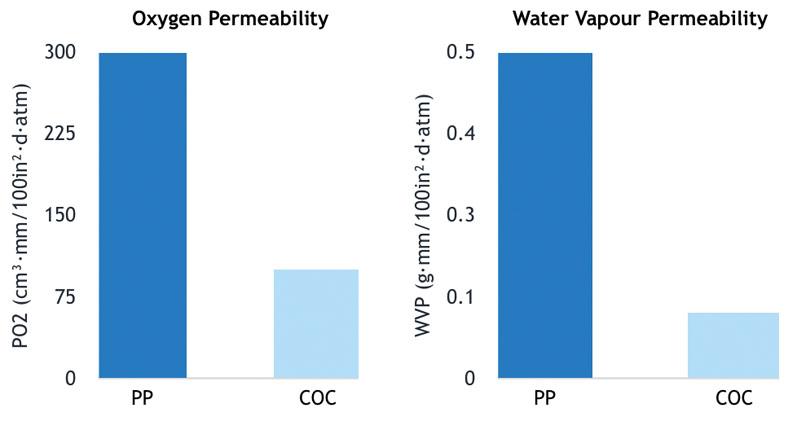
Figure 1: Comparison of the oxygen and water vapour barrier properties of a typical medical-grade PP and COC-grade TOPAS; the samples were analysed on a MOCON Multi-Tran 400 instrument using a TCD sensor11
Compared with PP, COCs have higher barrier properties, especially in terms of gases such as oxygen and water vapour (Figure 1). This is an important factor for hospital medications as oxygen is a leading factor in the loss of potency of a drug.
A barrel material with a higher oxygen barrier enables the filling of PFSs with a longer drug shelf-life. Another important element affecting drug stability is extractables and leachables. Silicone oil, which is typically applied to the inner surface of syringe barrels to enable a smooth plunger movement, is known to interfere with medications and to reduce drug stability.
PP syringes have a significantly higher amount of extractable silicone than COC syringes do (Figure 2). Furthermore, the silicone value fluctuates considerably depending on the syringe. The silicone level for COC syringes is lower and more constant.
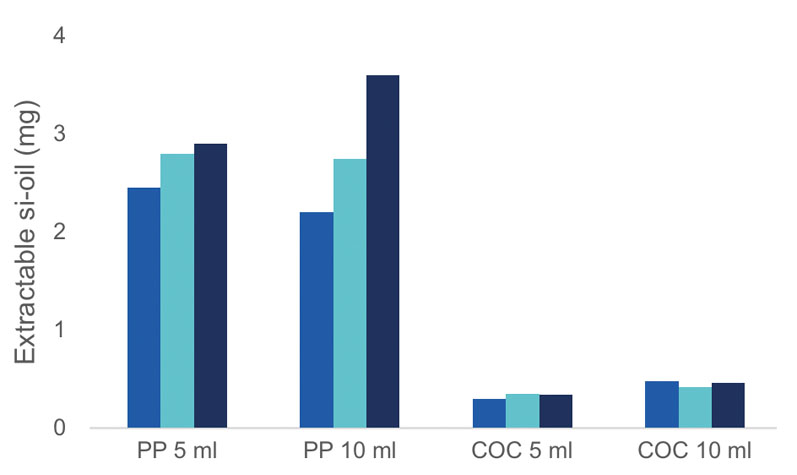
Figure 2: Comparison of the extractable silicone oil levels for PP disposable syringes (5 mL and 10 mL) and COC SCHOTT TOPPAC (5 mL and 10 mL) syringes; the total amount of silicone was extracted with heptane in an ultrasonic bath and the silicone level was subsequently determined with graphite furnace atomic absorption spectrometry (GFAAS)
SCHOTT TOPPAC infuse syringes meet the demands of the healthcare industry for prefillable polymer syringes that are ideal for infusion therapy in hospitals. The syringes are lightweight with a glass-like appearance and are available in a range of volumes (1–50 mL). They are compatible with a wide range of syringe pumps and IV devices.
The high barrier properties and low extractables and leachables profile of these COC syringes enable high drug stability and a long shelf-life. A PFS made of high-end polymer helps to reduce drug waste and microbial contamination while ensuring that the right amount of drug is administered in the right concentration at the right time, thereby reducing medication errors and improving patient safety.
The possibility of adding further features to COC PFSs in the future, such as smart labels, RFID chips, barcodes and tamper-evident technologies, will further increase patient safety and drive the use of PFSs in hospitals.
References
- P. Hinderliter and S.A. Saghir in P. Wexler (Ed.), Encyclopedia of Toxicology (Third Edition), Academic Press (London, UK, 2014).
- S. Berdot, et al., “Interventions to Reduce Nurses’ Medication Administration Errors in Inpatient Settings: A Systematic Review and Meta-Analysis,” Int. J. Nurs. Stud. 53, 342–350 (2016).
- www.healthcarepackaging.com/home/press-release/13282930/worldclass-glass-tubing-for-pharmaceuticals-100-years-of-schott-fiolax.
- www.fda.gov/regulatory-information/search-fda-guidance-documents/glass-syringes-delivering-drug-and-biological-products-technical-information-supplement.
- M.L. Colsoul, et al., “Long-Term Stability of Lorazepam in Sodium Chloride 0.9% Stored at Different Temperatures in Different Containers,” Hosp. Pharm. 55(3), 188–192 (2020).
- A. Jäppinen, et al., “Stability of Sufentanil and Levobupivacaine Solutions and a Mixture in a 0.9% Sodium Chloride Infusion Stored in Polypropylene Syringes,” Eur. J. Pharm. Sci. 19(1), 31–36 (2003).
- C. Anderson and M. MacKay, “Stability of Fentanyl Citrate, Hydromorphone Hydrochloride, Ketamine Hydrochloride, Midazolam, Morphine Sulfate and Pentobarbital Sodium in Polypropylene Syringes,” Pharmacy 3(4), 379–385 (2015).
- www.fda.gov/drugs/drug-safety-and-availability/fda-notifies-health-care-professionals-becton-dickinson-replaced-problematic-rubber-stoppers-its.
- www.sciencedirect.com/book/9781455732012/plastics-in-medical-devices.
- www.pharmaceutical-business-review.com/news/schott-pharmaceutical-packaging.
- https://topas.com/tech-center/performance-data/barrier-general.

