Small molecule therapies are created by multidisciplinary teams composed of chemists and biologists, whereas large molecule ones — proteins and antibodies — are almost exclusively developed by biologists alone (with the exception of antibody-drug conjugates [ADCs] for which the insights of scientists from both disciplines have been critical to their success).
This traditional segregation continues to dissolve as innovative drug products become more complex. Bringing these medicines to market, including drug development, GMP manufacturing and regulatory filing, requires deep expertise in both chemistry and biology.
Despite the rise of biologics, small molecule drugs continue to dominate the pharmaceutical market. The development of small molecules depends mostly on the expertise of biologists to identify novel modalities for therapeutic intervention and on chemists to design and synthesise structures with suitable activities and pharmaceutical properties to take advantage of them.
Biologists provide the assay systems and high-resolution structures of compounds bound to the target protein that allow for the prediction, testing and refinement of binding hypotheses, but it’s typically the chemistry team that designs each new generation of molecules and drives the development of the clinical candidate.
The quality of the collaboration and communication between the biologists and chemists of these integrated discovery teams is key to both their efficiency and likelihood of success.

By contrast, biologists have done most of the heavy lifting in terms of applying biotechnological advances to create monoclonal antibodies (mAbs) and other large molecule drugs. These complex molecules are designed using molecular biology techniques, rely on cell cultures and are purified and analysed by biologists ... with little chemistry involved.
Now, with the success of ADCs and the discovery of mRNA medicines, we are seeing the traditional segregation between the development pipelines of small molecules and biologics dissolve. For example, although mRNA is made in a test tube by combining DNA, nucleosides and enzymes in a chemical-like reaction, many of the ingredients are made using cell cultures — a process that is traditionally considered to be biotech — and the reactions themselves are classic biochemistry, but on a scale beyond that generally found in a non-GMP research setting.
The replicative enzymes are proteins and the DNA and resulting mRNA can be hundreds or thousands of bases long. Does this make an mRNA vaccine, encapsulated in a lipid nanoparticle (LNP), a small molecule, a large molecule or something altogether new? However they are defined, one thing is certain: the development, scale-up and commercialisation of these novel therapeutics requires the combined efforts of chemists and biologists.
Complex molecules need integrated R&D and manufacturing teams
The average cost of drug development is $1.3 billion and it can take up to 10 years to bring a new drug to market.1 But, with the speed at which the industry is moving, especially with mRNA and vaccines, pharmaceutical companies need to be doing things differently. Increasingly, biologists and chemists need to collaborate closely to get a drug to market.
An integral part of success during a drug’s lifecycle comes from combining robust R&D with the ability to scale-up to GMP production and regulatory filing, all in the same company. Most start-ups and pharmaceutical companies have to partner with multiple external parties to scale-up production and get their drug products to market.
During the past decade, Curia, formerly AMRI, has grown to be the kind of partner these companies need, offering consolidated services from early stage drug hits in a medicinal chemistry lab all the way to commercial manufacturing.
Curia’s acquisitions of LakePharma and Integrity Bio have expanded the company’s capabilities to include large molecule drug discovery and the development, manufacture, formulation and fill/finish of advanced therapies, including proteins, antibodies, ADCs, LNPs, viral vectors and mRNA drug products.
Collaborative strength in both chemistry and biology
Curia now has across-the-board expertise in the various technical areas required to produce mRNA therapeutics, ADCs or other biologics. These capabilities, when combined, provide customers with an end-to-end offering from early discovery to fill/finish. Each step is supported by teams of chemists, biologists and quality and regulatory specialists numbering in the hundreds at multiple locations throughout Curia’s global network of excellence (Table I).
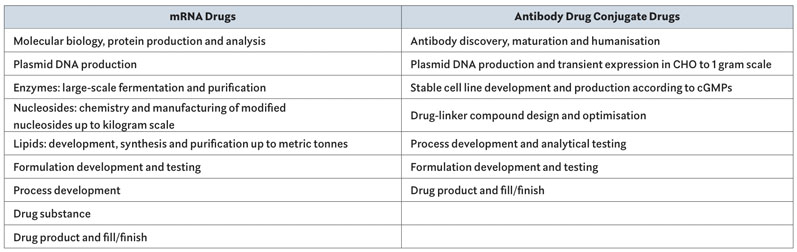
Table I : Capabilities: All steps of the development and manufacturing process are covered by multiple teams in Curia’s global network of 30 facilities
From mRNA to LNP: global capacity to produce novel therapeutics
Manufacturing an mRNA-LNP product, such as a vaccine, is a complex process, beginning with the in vitro transcription of mRNA and ending with that mRNA being encapsulated in an LNP in a finished drug product ready for patients. With complete analytical capabilities and an established raw materials supply chain, Curia is capable of every step of the production of mRNA drugs, including custom gene synthesis and plasmid DNA production.
The creation of modified nucleosides, mRNA transcription and the large-scale production of specialised lipids, as well as mixing mRNA with lipids to form the LNPs that are essential to the formulation of these novel drugs, also falls within our remit (Figure 1).
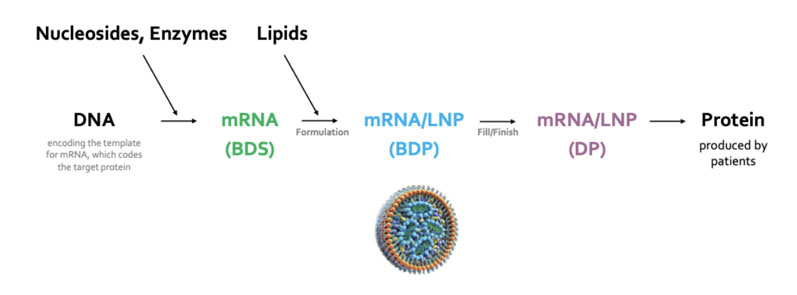
Figure 1: The main steps in the process of making an mRNA therapeutic (BDS = bulk drug substance, BDP = bulk drug product, LNP = lipid nanoparticle, DP = drug product)
Although much of the focus in the popular press is on the mRNA portion of these new drugs, the most technically difficult steps are the production of lipids and the formulation of the LNP. Curia has extensive expertise in the development, scale-up and effective purification of numerous lipids, including the metric tonnes of lipids it manufactures at its GMP facilities in Europe.
Its operational flexibility and co-ordination between multiple sites have allowed the company to become a major supplier of critical lipids used in the formulation of approved mRNA drug products.
Bioconjugation and ADCs
Bioconjugation — as used to manufacture ADCs — is a process in which the chemistry of small molecules meets the biology of large ones. Ideally, a team will have a near-equal contribution of chemists for the creation of warheads and linkers, and biologists for the design, production and analysis of mAbs and ADCs.
Curia has traditionally made warheads, linkers, drug-linker compounds and custom ADCs using mAbs provided by clients. Now, with the acquisition of LakePharma, Curia has the discovery, development and manufacturing capability for mAbs and other forms of therapeutic protein.
A pharmaceutical manufacturer can supplement its internal resources with outsourced expertise to fill gaps but, when chemists and biologists are part of the same company, they can collaborate side by side without a confidential disclosure agreement (CDA), making the process faster and more efficient.
Many of the groundbreaking medicines of today require a range of complex production processes, from custom gene synthesis, molecular cloning, chemical design and synthesis to large-scale fermentation for protein production. The most efficient and cost-effective way to bring these products to market is to work with a partner that has large, integrated teams of both chemists and biologists.
Reference
- O.J. Wouters, M. McKee and J. Luyten, “Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018,” JAMA 323(9), 844–853 (2020).
