Drawing of a small spherical liposome seen in cross section
Source: Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002
Liposomes are valued delivery vehicles for potent and efficacious active pharmaceuticals. Due to their architecture mirroring a cell, and the added provision of being able to modulate active ingredient bioavailibilty while reducing detrimental consequences, they are highly favoured as vehicles for macroscopic drug delivery along with peptides, siRNA etc. The means of manufacturing liposomes via a method which offers a reliable, repeatable, controllable and scalable measure is therefore highly sought after.
Why utilise high pressure homogenisation (HPH)?
The HPH process offers rapid reduction of vesicle size and lamellarity to achieve smaller vesicle sizes whilst narrowing the distribution. The aim is to:
- Develop the macroscopic appearance of the preparation
- Increase physical stability preventing aggregation, sedimenting or solution crash out and forcing liposomes to float
- Produce a filterable preparation allowing passage through the microbe-retentive filters
Reducing the vesicle size presents a distinct biological advantage as it is less easily lost from the bloodstream, moreover these macromolecules stay sterically stabilised.
How is this achieved?
It is widely known that the process of homogenisation at such high pressures leads to particle size reduction, two mechanisms are primarily responsible:
- Primary vesicle size reduction
- Reducing number of vesicle aggregations
- Homogeniety of the initial product
- Homogeniser design
- Processing pressures and number of passes
- Temperature
- Lipid composition and content
- Ionic strength of the medium
- Ingredients degrade: the strong cavitation and shear forces generated and intense energy the lipids are subject to. As an example, molecules such as insulin and enzymes, although able to withstand the forces exerted by the homogenisation process, are heat sensitive which means that the homogeniser used has to offer complete sample temperature control.
- Lipid layer is lost: Materials used in the construction of the systems, at times, also cause interference by causing adsorption of the lipid on the walls of the system.
- Erosion of the homogeniser: With increasing complexity of the architecture of the systems additional components in the units such as O’rings are responsible for contamination of the liposomal mixtures.
- Neurodegenerative, eg. Alzheimers, MS
- Cancers
- Cardiovascular disorders, eg. hypertension
- Infectious disease caused by bacteria, fungi etc.
Homogenisation of multi-lamellar mixture liposomes produces a uni-lamellar mix. After the first processing cycle, a significant drop in vesicle size can be observed with approximately only one concentric lamellae/vesicle. Regular process cycling ensures the distribution is tight and this observation is maintained throughout.
Loading of liposomes by homogenisation
High pressure homogenisation also presents the opportunity to load the liposomes, locating hydrophilic active substances within the aqueous vesicle core. The development of pharmaceutical drug delivery and food technologies sees the use of this (see Figure 2). Amphiphilic and lipophilic compounds can also be encapsulated with liposomal vesicles through the means of high pressure homogenisation, where it may be dissolved in an organic solvent with lipids and hydrated upon solvent removal pre-homogenisation.
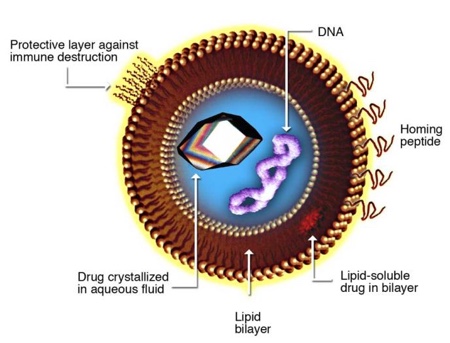
Figure 2: Liposomal design for drug delivery
Source: Torchilin, VP “Multifunctional Nanocarriers.” Adv Drug Deliv Rev 2006 Dec; 58 (14): 1532-55 doi: 10.1016/j.addr.2006.09.009
Abraxane, a liposome-based drug by Abraxis Bioscience, was recently modified via high pressure homogenisation to combine albumin and paclitaxel, allowing free binding and unbinding within the blood stream, presenting greater amounts of unbound drug in the blood stream. Gemcitabine, marketed as Gemzar by Eli Lilly, is also a widely known and utilised as cancer treatment for a variety of carcinomas. A liposomal formulation of this drug GemLip is currently being developed to be presented as intravenous injection.
Impacting factors on liposomal size
Vesicle size and the success of the homogenisation process is greatly influenced by the following factors:
These factors need to be controlled carefully to ensure the final preparation is stable as well as potent. Design of the homogenising chamber also plays a role in the effectiveness and even distribution of pressure on the vesicles. Higher pressure can yield smaller sizes, though at a cost of heat generation and increased energy expenditure.
Avestin offers a control on both the pressure and the cycling to allow for both reduction in the polydispersity of the sample and the average liposomal size. In general, after one pass, a bi-modal particle size distribution can be seen with a propensity to slowly skew left with repeated passes. However, the combined high pressure homogenisation-extrusion that the Avestin systems offer, reduces the amount of cycling required thus offering a faster and more effective and reliable means of process (see Figure 3).
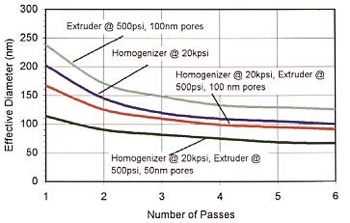
Figure 3: Combined homogenisation/extrusion led to liposomes with a particle size of 113.7nm in one pass and 89.9nm in two passes. The effective diameter was measured using the Brookhaven Instruments 90Plus Particle Size Analyzer
Other factors to consider in achieving successful liopsomal manufacture
In addition to the critical factors mentioned above, there are a few other elements that need to be considered in achieving successful liposomal manufacture.
The Avestin HPH range presents a controllable means ensuring even the most delicate substances are catered for, with complete temperature, pressure and cycle control. In addition, the simplified design of the units (which are also FDA approved) ensures issues of material interference is circumvented, and the product integrity is maintained.
Shelf stability of homogenised liposomal mixtures
Though composition dependant, high pressure homogenisation is shown to offer promising periods for long-term shelf life, with vesicle sizes and stability being maintained for an average of 18 months. Enhancements in shelf-life following homogenisation can be made with freeze drying. This offers a completely contained process for drug manufacture including liposomes encapsulated with active ingredients.
Some of the conditions utilising this technology include:
Avestin offers several options from extrusion, to high pressure homogenisation and the cutting edge combined high pressure homogenisation-extrusion system to manufacture liposomes at a nanoscale for extremely delicate compounds with scalability up to 1000L/hr.
If you would like more information on how high pressure homogenisation can help your projects, please contact Melvin Jose on mjose@biopharma.co.uk or +44 (0)1962 841092.
Bibliography
Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002.
Liposomal design for drug delivery. Source: Torchilin, VP 'Multifunctional Nanocarriers'. Adv Drug Deliv Rev 2006 Dec; 58 (14): 1532-55 doi: 10.1016/j.addr.2006.09.009
Fischer. B . Paul, Tew. D. K(2015) Advances in Cancer Research
Bornmann C1, Graeser R, Esser N, Ziroli V, Jantscheff P, Keck T, Unger C, Hopt UT, Adam U, Schaechtele C, Massing U, von Dobschuetz E. (2008) A new liposomal formulation of Gemcitabine is active in an orthotopic mouse model of pancreatic cancer accessible to bioluminescence imaging. Cancer Chemother Pharmacol
Sorgi F.L and Huang L, (1996). Int. J. Pharma., 144, 131

