Before the COVID-19 crisis, biopharmaceuticals were already focused on capacity. The BioPlan Associates 16th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production asked the following question in 2019: “If this industry is to avoid significant capacity constraints, the most important areas to be addressed are …?”
There were marked differences between the US and Western European responses: 42.2% of Western Europeans focused on developing better downstream purification technology compared with 28% of US participants; 45% of US respondents would develop better continuous processing technology compared with 33.3% of Western Europeans and both groups saw new single-use facilities as important.
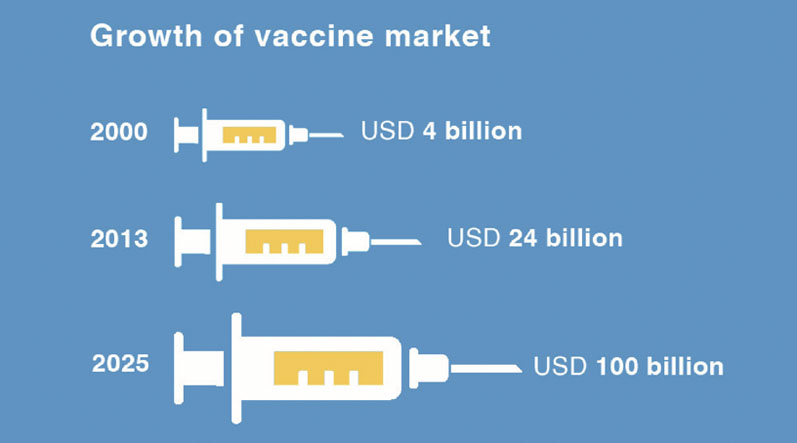
COVID-19 has amplified this need. Capacity is now under strain and the scale of this production effort must not be underestimated. To produce the billions of vaccines needed to fight this pandemic, there will be a huge demand for materials.
Producing enough drug product doesn’t matter if the delivery devices aren’t there. To meet the requirement for all aspects of vaccine production, we will likely see innovative resource reallocation and pioneers from other industries lending a hand, just as we did with Dyson ventilators and the pop-up 3D printing firms making face shields for frontline health workers.
The many and diverse vaccines entering the COVID-19 race can only be a good thing. No single biopharmaceutical or research institution can manage the worldwide production of a single vaccine. There are likely to be multiple winners in this race and, through collaboration with different CMOs and suppliers, the production and demand for raw materials can be dissipated.
Although WHO planned for a co-ordinated effort, success will be seen in the smaller collaborations formed between industry leaders in their areas of expertise.
Could collaboration lead to the UK’s first COVID-19 vaccine?
We’re already seeing organisations use their collective brainpower and experience to tackle the COVID-19 problem. Collaborations including academic institutions, Big Pharma, smaller biotechs, CROs and parterships such as GSK and Sanofi, AstraZeneca and Oxford University, and Merck and IAVI, and are already delivering promising early stage results as vaccines reach clinical trials.
Tony Hitchcock, Technical Director, Cobra Biologics, explains how collaboration is driving this development. “Cobra Biologics’ background is traditionally in gene therapy, taking preclinical trials through to GMP-approved clinical manufacturing. Our experience in working with viral vectors lends a particular strength to vaccine manufacturing."
"Although vaccines have been universal in responding to endemic diseases, COVID-19 is a completely new scenario for us all. Thankfully, we can draw on related understanding to mount a strong and collective assault on this virus.”
“What’s powerful about several of these vaccine approaches is the use of well-established technologies and processes to expedite the development,” he continues: “The vaccine development timeline has already been reduced from 5 years to 4 months by utilising existing platform technologies, such as the chimpanzee adenovirus platform that was established more than 15 years ago and was used to develop SARS and MERS vaccines.”
“Although these vaccines couldn’t enter clinical trials as the levels of infection dropped, we already have important dosage and efficacy data as the technology was tried, tested and approved for use in vaccine development. This is true for much of the manufacturing process too."
"By utilising single-use technology at the preclinical stage that can be pulled through to the scale-up stages, existing validation documentation can be used to prove the effectiveness of the manufacturing process.”
“The strength in this approach lies in its simplicity. Through tried and tested bioprocessing techniques, we can produce a stable and effective vaccine. Once the vaccine is proven to work in clinical trials, we can quickly scale-up this process to produce millions of vaccines."
"When responding to a pandemic, the tried and tested techniques work. Although there are more innovative techniques being developed, such as plant, RNA and virus-like particle (VLP) vaccines, we simply haven’t yet established techniques to scale these processes.”
Clinical trial volumes for vaccine candidates will need to be manufactured at multiple sites simultaneously to provide the required volumes across several locations.
“This model of scaling-out production can be replicated anywhere in the world using the agreed processes and specified single-use technology."
"The technology must be the same in every manufacturing site, which is how we’ll achieve process validation and meet the regulatory standards. Mapping a critical path to ensure a safe and secure supply of consumables and protect the long-term production of these vaccines is also essential, so the relationship between developers and bioprocessing partners is crucial.”
“Although vaccine production is traditionally isolated from that of other biologics, such as monoclonal antibodies and cell and gene therapy, the process uses much of the same equipment and requires a similar skill base. As an industry, we have access to both, making large-scale production a reality.” “Collaboration across multiple organisations is clearly important, with each partner bringing unique expertise to the project. Hopefully, based on the very real and solid foundation of tried and test technology, these collaborations will create something truly groundbreaking.”In conclusion
Some of the world’s brightest minds have been working on the COVID-19 crisis and the dedication and hard work of scientists, engineers and manufacturing teams will surely bear fruit. Science is an evolution. Just as we’ve used learnings from SARS and MERS vaccines to fight this pandemic, so too will we learn from COVID-19 vaccine development.
The advancements to accelerate vaccine production, intensify processes and scale-out production will set the scene for even greater development in these areas. But, most of all, collaboration will be the lasting reminder from this crisis.
Biopharmaceuticals, CMOs and suppliers — including competitors — have come together with a common purpose. We will have cost-effective vaccines against COVID-19 and we will have them soon.
As we move towards a challenging future, an ageing population requiring a diverse range of medication, a push towards personalised medicine and the more likely emergence of other novel viruses and future pandemics, we stand firm in the knowledge that we can meet these challenges with a blend of innovation and established solutions.
Part I of the article can be found here.
