Tracking parts right back to the manufacturing data has taken another smart leap forward, with moulding machine specialist Sumitomo (SHI) Demag pioneering a fully automated In Mould Decorating (IMD) production cell. It can be used by moulders to issue medical devices with globally compliant Unique Device Identifications (UDIs).
With the introduction of the new EU Medical Device Regulations (MDR), every device or its packaging must be issued with a Unique Device Identification (UDI). Given that there are just 18 months until the MDR directive become mandatory, the cell tackles what some in the industry perceive to be a challenging and costly undertaking.
Designed predominantly for healthcare, automotive and aerospace manufacturing environments where quality control and traceability are critical, the advance represents a colossal change in how multiple components are individually issued with a unique identifier.
Nigel Flowers, Managing Director at Sumitomo (SHI) Demag UK, likens it to issuing each moulded medical device component with its unique birth certificate, with all processing data held securely by a manufacturing executive system (MES).
It means that any potential quality defect, which might not be picked up for several months, or even years, can be tracked back to the very day and cycle it was manufactured to achieve item-level traceability and conduct root cause analyses on parts and components.
Putting this into context, Nigel emphasises that authentication of individual components in high liability markets like healthcare requires a fingerprint style approach to traceability. In the interest of patient safety, MDR requires that a UDI label be directly attached to a medical device or to its packaging.
Additionally, labels in the future will need to include two identifiers: a device identifier (DI) that identifies the labeller and the specific version or model of a device, plus a production identifier. This variable portion of the UDI needs to include the given lot or batch number, serial number, date of manufacture, expiry date, etc.
Meeting the new directive ahead of schedule
Nigel explains: “Heavily regulated products like medical devices require comprehensive audit trails. However, it is not purely about mandatory information and supply chain tracking. Real-time traceability is about being able to call up data and verify the exact settings used on the injection moulding machine when that individual plastic part was made. That’s where connectivity to a Management Executive System (MES) is vital.”
By tightly integrating all elements of the plastic injection processing, IMD, robotics and data capture and management into a single turnkey cleanroom cell, part-specific traceability and process monitoring is enhanced.
It represents another step towards the Smart Factory for tier 1 to 4 medical device injection moulders, offering heightened risk management, mitigation and containment, which enables organisations to respond accurately and rapidly with a targeted recall.
Using robotics is central to the inline manufacturing process, as even a single instance of applying an incorrect code can be a major liability cautions Nigel.
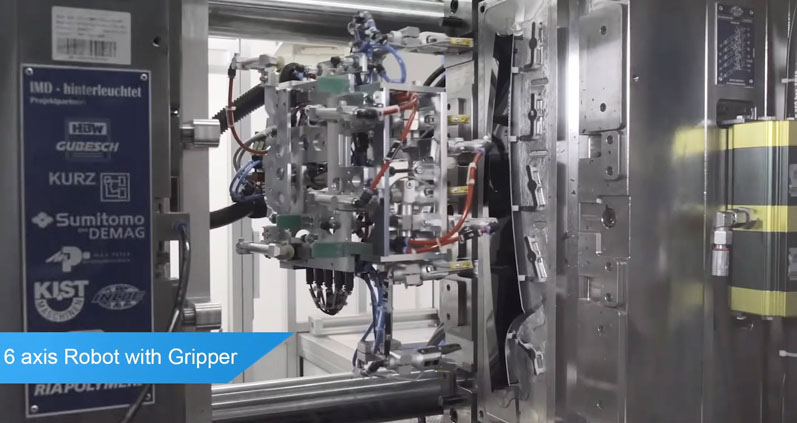
To eliminate the risk of the wrong QR code being applied to the medical device component, Sumitomo (SHI) Demag deploys a highly dextrous six-axis robot to remove each part from the mould. The robot holds the part during the entire time the QR code is etched on at the laser marking station, never releasing until the data has been scanned and stored in the holding data system.
“What we have created is a fully automated plastic injection moulding and laser marking station that connects and communicates the code back to MES holding system where it reconciles up with the machine processing data. In addition to boosting traceability, this synchronised data gives production managers greater visibility on product cycle times and quality,” adds Nigel.
Previously siloed manufacturing phases can now be fully integrated by leveraging Industry 4:0 technology, notes Nigel.
To illustrate the potential possibilities, Sumitomo (SHI) Demag, in partnership with KURZ, recently unveiled a new online video showcasing the making, marking and quality check of a highly innovative automotive door trim with a high gloss day/night design.
The technique used for this automotive application would be just as, if not more so, relevant for medical device manufacturers seeking a solution that complies with the coding of medical parts for MDR, highlights Nigel.
The fully automated Systec Servo cleanroom cell, which features a 6 axis robot with gripper, also appeared at Fakuma 2018, and breaks down the individual production steps involved in creating a super-sleek day/night design car door trim in less than 50 seconds.
Once decorated using advanced IMD, foil is then added to the door trim panel during the bonding process to provide touch functionality. Process data is then integrated into the higher-level MES to generate the unique data matrix sensor, which is bonded to the part to give each individual part its traceability data.
In addition to the exact production date and time, process data that’s recorded includes the injection and dosing time, melt cushion, injection pressure and temperature.
Helping to avoid costly recalls, this enhanced traceability enables organisations to respond more effectively should a defective medical device part or component enter the value chain.
Nigel comments: “Owing to globalised production platforms, it’s becoming increasingly imperative to limit operational risks exposures with targeted rather than mass recalls. For high value components or when the margin for error is zero and patient safety could be at risk, real-time traceability provides the means to limit recall exposure by improving end-to-end process transparency.”
Trade association MedTech Europe estimates that there are around half a million different medical devices currently available in the EU, with medical technology accounting for 7.2% of total healthcare expenditure. The new MDR European regulations are closely aligned to US guidelines issued by the Food and Drug Administration (FDA).
