Advances in drug discovery technologies and combinatorial chemistry techniques have led to identification of a number of compounds with good therapeutic potential. However, the number of insoluble drug candidates has increased in recent years with almost 70%1 of new drug candidates showing poor aqueous solubility, which can result in reduced and variable bioavailability.
Drug nanoparticles
One avenue gathering popularity for compounds with minimal or no solubility is drug nanoparticles. Altering size from the micron to the nm range results in a significant increase in surface area: for example, when the particle size of a drug is condensed from 8µm to 200nm there is 40-fold increase in the surface area to volume ratio. This results in a substantial amplification of the dissolution rate, if the formulation disperses into discrete particles.
Nanoparticle formulation technologies therefore provide the pharmaceutical industry with options for addressing issues associated with poorly solubility. In new chemical entities (NCE) development, the technology has been of great value when it is used as a screening tool during preclinical efficacy and/or safety assessment studies in the early development phase. For marketed products requiring lifecycle extension opportunities, nanoparticle formulation strategies provide a means to develop a new drug delivery platform with improved therapeutic outcome incorporating the existing drug, thus creating new avenues for addressing unmet medical needs.
Nanoparticles tend to be developed by two distinct set of technologies: ‘bottom up’ and ‘top down’. Bottom up technologies focus on the use of non-solvents in a controlled precipitation and crystallisation procedure to create complex assemblies in a useful conformation, through the self-assembly of molecular sized components.
Pharmaceutical firms were quick to implement these techniques in the race to develop successful nanoparticle- incorporated formulations for poorly soluble potent compounds. Novartis and Abbott laboratories were among the first2 to implement these in their R & D for the development of hydrosols and carotene nanoparticles respectively. Though useful these techniques soon dropped sharply in popularity within the industry to the current point of not being used at all in the production of commercial products.
This loss of popularity of ‘bottom up technologies’ was due to a range of issues, from the need for solvent removal to the difficulty in controlling the process, and the fact that many poorly soluble drugs are poorly soluble not only in aqueous, but also organic media.
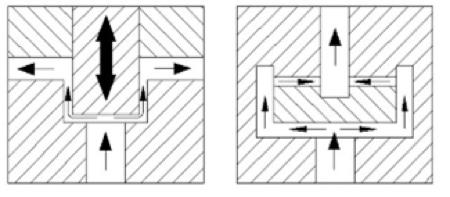
Figure 2: Dynamic (left) and static (right) homogenising valve principles
Technique of choice for particle size reduction
Top down techniques involve homogenisation to reduce particles to homogenous sub-micron size, which together form a stable and potent entity. Particle size reduction has been reported to improve the dissolution rate and bioavailability of several poorly water soluble drugs such as spironolactone, budesonide and omeprazole by effective size reduction to the nm size range.
High pressure homogenisation (HPH) is seen as superior to other top down technologies such as milling, being a more feasible means for both laboratory scale and industrial manufacture. High pressure homogenisation has also been known to avoid other drawbacks such as amorphisation, polymorph transformation and metal contamination, which result from the high mechanical energy associated with conventional milling processes. Therefore this technology in particular is beneficial for comminution of drug particles where the importance of repeatability is prominent.
The core technology in HPH
HPH forces the drug powder and surfactant mix through a miniature orifice while subjecting it to pressure from 35 to 2000 bar (500 to 30,000psi). The resulting high streaming velocity of the suspension causes an increase in the dynamic pressure which is compensated by a reduction in the static pressure below the vapour pressure of the aqueous phase, resulting in cavitation and high shear forces with turbulent flow.
This process of drug particle size reduction needs complete control over a number of process parameters including the homogenisation pressure, number of homogenisation cycles and the concentration of the stabilising agent.
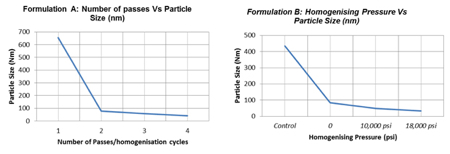
Figure 3: Two different nanoparticle pharmaceutical emulsions prepared using the EmulsiFlex high pressure homogeniser3
Of the three, the homogenising pressure has the largest impact on the particle size reduction and distribution. Higher pressures provide the highest particle size reduction and distribution. However, to achieve a stable and potent drug, especially in the presence of a surfactant, the relationship between pressure and number of cycles needs to be considered.
A lower pressure with a higher number of passes may be enough for some products to achieve the same stability and particle size as a higher pressure with less passes.
The driving force for industrial development
High pressure homogenisation offers an effective means to overcome solubility issues in chemically complex drug formulations. The Avestin range of high pressure homogenisers presents a scalable technique for drug development and industrial manufacture.
References
1. Fundamentals of Pharmaceutical Nanoscience by Ijeoma F. Uchegbu, Andreas G. Schätzlein, Woei Ping Cheng, Aikaterini Lalatsa 2013, Page 278
2. Nanoparticulate Drug Delivery Systems Volume 166 By Deepak Thassu Michel Deleers Yashwant Vishnupant Pathak – March 30, 2007 page 78.
3. Information provided by Avestin, Inc

