Gene therapies play an increasingly significant role in the future of medicine. Process development and quality testing are yet in the focus of the international authorities. Today, due to their specific characteristics as non self-replicative viral vectors, adeno-associated viruses (AAVs) are a safe approach for delivering the genes of interest.
Innovative companies face analytical challenges to find the most effective ways of process development and standardisation. There is a broad range of important quality attributes for delivery vectors which need to be closely controlled for efficacy and biosafety of gene therapy. PPS has established specialised testing packages for the characterisation and quality control testing of AAV particles and constituent capsid protein assemblies.
Adeno-associated viruses – benefits and challenges
AAV based gene therapy is driving today’s therapeutic discoveries and has evolved to the leading strategy for the treatment of genetic diseases. Simultaneously, the regulatory demands are increasing and authorities request extensive analytical characterisation for the drug vectors (e.g. capsid loading, process impurities). Hence, reliable, robust, GMP-compliant analytical methods and characterisation protocols are required. Specific analytical assays need to be performed to assess vector productivity, vector purity, biological activity and safety.
Analytical toolbox
Based on more than 20 years of analytical, biopharmaceutical experience, we can address our clients’ specific needs by swift and effective analytical support throughout the whole lifecycle of their gene therapy products and development processes. As a full-service partner PPS offers a broad range of analytical methods to quantify the strength/dose of the AAV vector, to quantify full and empty particles as well as particles carrying wrongly encapsulated DNA. Therefore, our analytical platform enables us to ensure the identity, purity, potency, and safety of the final product. To prove whether the gene of interest is present in the vector is essential for surveilling the production process as well as for the evaluation of the right dosage for the patient.
As a very common example - PPS can provide information on vector loading using several techniques:
- Analytical ultracentrifugation (AUC)
- Size-exclusion chromatography combined with multiangle laser light scattering (SEC-MALLS)
- Capillary gel electrophoresis assay (cGE)
- Ion exchange chromatography (IEX)
AUC analysis in particular can be integrated in existing processes to detect vector loading at particular steps in manufacturing and by that constributes to the quality of the final product.
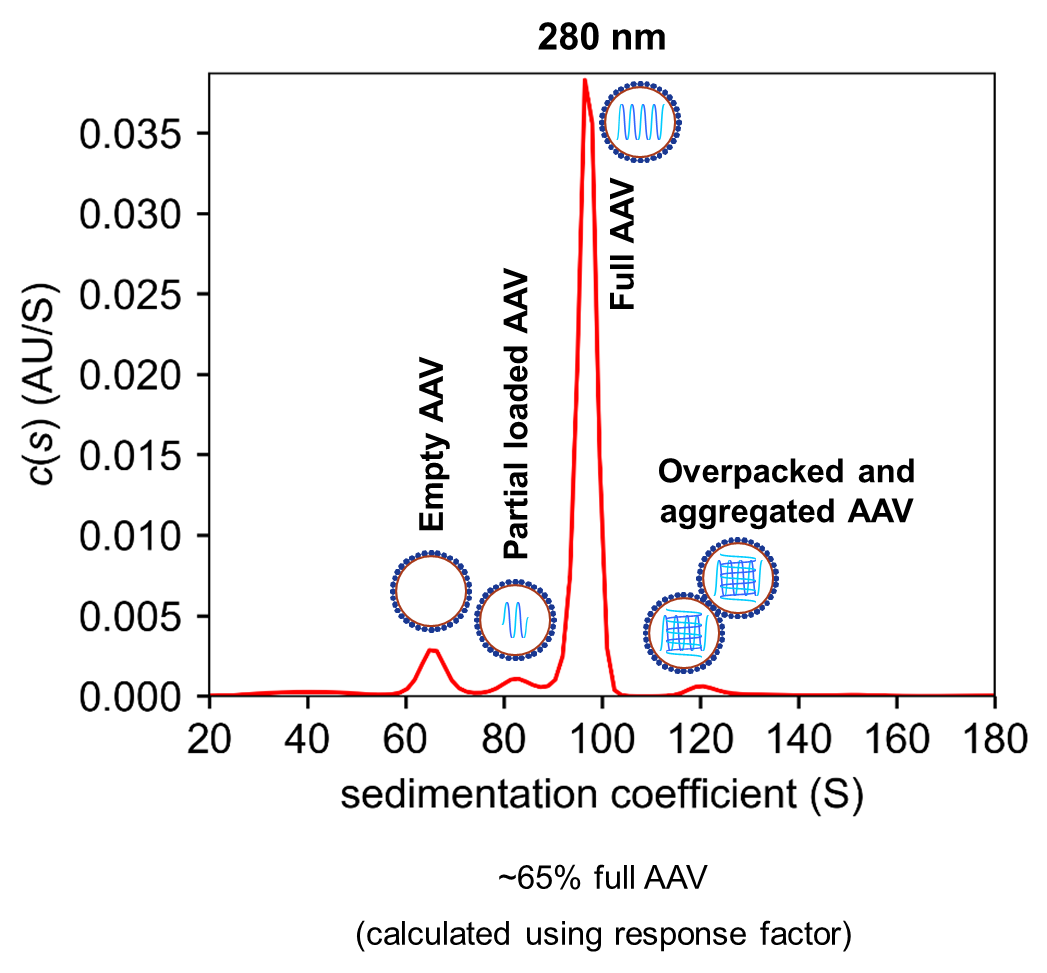
Figure 1: Analysis of capsid loading of AAVs using Analytical Ultracentrifugation (AUC)
"Quality control of adeno-associated virus preparations can be a very complex matter in respect to the viral vector/serotype or the formulation compounds," said Dr Stefan Haberland, Teamleader Biotherapeutical Analytics. "We use analytical ultracentrifugation not only to analyse the relative amounts of filled and empty capsids in an AAV preparations, but also to identify partially filled and overpackaged species."
SEC-MALLS allows testing several critical quality attributes of an AAV product.
Dr Haberland explained: "Our SEC-MALLS platform provides data to measure different parameters within one single run, such as molecular weight, capsid-particle concentration and empty-to-full ratio. They can easily be implemented into the established production processes as an orthogonal and complementary approach that offers data validation throughout the whole AAV production process. An indispensable and time-saving tool for AAV manufacturing, quality control, and assurance of regulatory aspects."
Quality control along the development and production pathway
By means of our comprehensive portfolio we support the development and execution of cell- and gene therapy programs from basic research through to the clinical stage by providing innovative, safe and GMP-compliant CMC testing. Our technology ensures the safe and reliable analysis of identity, potency, purity, and safety of starting materials, intermediate products, vectors and final drug products as well as support for manufacturing process development and validation.
From mass spectrometry (in vivo cQA mapping, peptide mapping, molecular weight determination, glycan profiling) through to higher order analysis (DLS, DSC) and analytical testing (e.g., AUC, LC-MS, HPLC, ELISA and qPCR methods, UPLC)
