The very first step in a drug development project is studying its feasibility. An initial proof of concept is key to evaluating crucial aspects such as the characteristics of the active ingredient, the drug product performance and/or the compatibility of the formulation.
Capsules facilitate this process as it’s very easy to simply fill a capsule with a dose of the active pharmaceutical ingredient (API).
There’s no need for complex equipment as capsules can be handled using a small-scale manual or semiautomated filling machine at this stage.
In this sense, hard capsules reduce the time-to-market and eliminate the need to make a formulation or machinery investment during early phase development compared with other dosage forms.
Hard capsules also enable the development of a wide variety of delivery systems. From the most standard form consisting of a dry powder filled into a capsule to combined formulations of pellets, softgels or multi-tablets inside the capsule, this versatility allows formulators to achieve the desired pharmaceutical action in a more time-efficient way.
Furthermore, drug targeting has previously led to molecules with very poor solubility: 60–70% of new chemical entities are Biopharmaceutical Classification System (BCS) Class II.

Formulators can overcome many API challenges using lipid-based formulations, especially when appropriate excipients are used to improve solubility and absorption.1
Liquid-filled hard capsules enable formulators to use many APIs that would be difficult or even impossible to incorporate into other solid dosage forms.2
Hard capsules can also be used for more sensitive active ingredients; HPMC capsules, with their low moisture content, offer a premium option for more complex drugs by providing high chemical stability and physical inertness.
They also prevent compatibility issues and brittleness in unexpected environmental conditions, thus ensuring positive stability results even when formulating with hygroscopic drugs.
Nevertheless, the dissolution profile of HPMC capsules is equivalent to that of traditional gelatin capsules. Additionally, hard capsules are not only restricted to oral administration; they can also be used for inhalable therapies.
Here, the hard capsule plays a critical role as a receptacle for the fine powder that’s delivered to the lungs by a dry powder inhaler (DPI). This sophisticated delivery system is becoming increasingly common, not just for respiratory diseases, but also for the treatment of systemic conditions such as diabetes and Parkinson’s.
Clinical studies
For the preclinical phase, tiny hard capsules can be used on conscious animals without stressing them, all while guaranteeing that a correct dose is delivered to the stomach.
Hard capsules also enable a wide range of doses to be used, bringing greater flexibility to Phase I studies. Besides, as various colours and sizes are available, they are extremely well suited for preliminary drug studies and blinded clinical trials.
Considering all the aforementioned points, there are several ways that hard capsules expedite time-to-market:
- they are simple to manufacture at small-scale
- they can be filled with neat API, if required, to rapidly demonstrate proof-of-concept and decrease the use of costly materials
- in terms of API stability and the capsule shell, there is little chance of incompatibility.
Manufacturing process
At the manufacturing stage, there are also many advantages when using hard capsules. As previously mentioned, the use of hard capsules enables a wide variety of drug delivery systems to be employed.
Hard capsules generally require fewer excipients and, therefore, fewer process steps and analytical tests, which accelerates both validation and CMC (Chemical, Manufacturing and Control) activities and facilitates scale-up.
In addition, there are fewer manufacturing steps when producing hard capsules — compared with tablets — as processes such as granulation, drying, compression and coating are avoided. In fact, the production of pellet-filled hard capsules can be reduced to just two steps: weighing and filling.
As such, working with hard capsules enables a reduction in manufacturing steps, production timelines and costs.
Hard capsules also demonstrate advantages in terms of operator safety when manufacturing products formulated with highly potent APIs. Compared with tablet production, there’s much less risk of API exposure as hard capsules function as hermetic containers.
Additionally, hard capsules with liquid and semisolid contents produce minimal airborne dust, once again optimising worker safety.
Marketing and commercialisation
One of the key aspects when marketing a drug product is brand recognition. Tablets can be film coated in many different colours, but hard capsules offer a wide range of possibilities in terms of product design, from using different cap and body colours, on-capsule printing or the ability to choose between a clear, opaque and/or even shiny appearance.
This can make products more attractive and easier to identify for patients, especially elderly ones, and it also helps medical staff to distribute the correct medications — also improving patient safety.
In the consumer healthcare market, there is a growing demand for organic, vegan and vegetal products.3 HPMC hard capsules enable the use of food supplements, for example, that comply with these labelling requirements.
Furthermore, from a regulation point of view, hard capsules contribute to anticounterfeiting measures as they are comparatively difficult to copy.
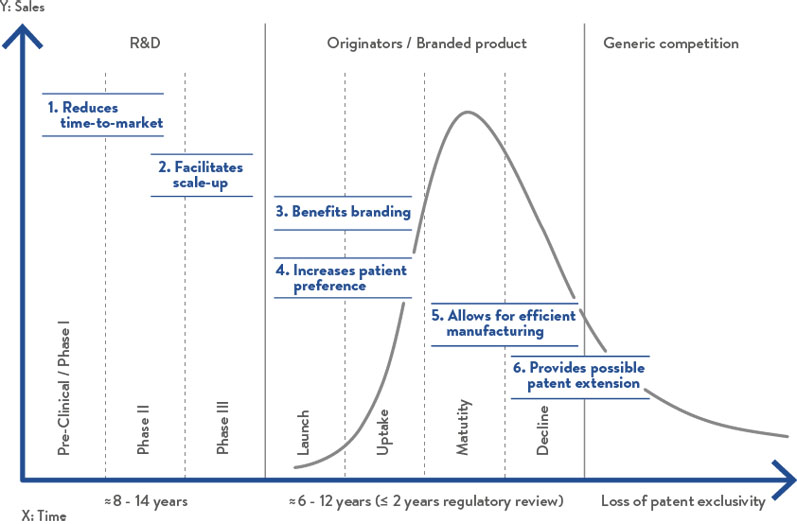
Figure 1: The benefits of hard capsules throughout the entire drug product lifecycle
Patience preference
In addition to everything described previously, one of the most important aspects of a pharmaceutical or consumer healthcare product is patient adherence.
Patients prefer capsules instead of tablets; they’re easier to swallow (optimal shape) and have a higher “perceived efficiency.” Patients believe that capsule-based APIs are more stable and act quicker. Besides, the capsule shell also masks unpleasant tastes and odours, is less prone to breakage, allows tamper-proof banding and is thought to be gentler and induce less gastrointestinal irritation than tablets.4
Regarding ease of swallowing, a study conducted by Hermes Pharma involving 2000 German and US consumers showed that 32% of respondents had broken up a tablet or crushed it (17%) before dissolving it in water prior to swallowing, whereas only 6% had emptied a capsule to take its contents.5
This data clearly demonstrates that some behaviours (when self-administering medicines) can lead to altering the state of the dosage form, which negatively affects the API release profile, bioavailability, dosing and efficacy.
Furthermore, owing to the versatility that hard capsules offer, formulators can combine multiple APIs in a single capsule. This allows combination therapies that reduce the number of pills to be taken by the patient.
Regarding inhalables, capsule-based DPIs are breath actuated, so patients don’t need to press and inhale at the same time, making them more user friendly.
In addition, the use of transparent capsules for inhalation drugs allows patients to verify powder emptying, ensuring that they’ve taken the whole dose. All these benefits contribute to enhanced patient adherence, which is the ultimate objective of each and every stakeholder in the healthcare system.
Conclusion
As discussed, capsules are a very versatile delivery system that can be optimised for a wide variety of formulations. Faster R&D activities and the rapid demonstration of proof of concept contribute to reduced times-to-market.
In addition, there is less cost and time impact throughout the development phase and scale-up. Plus, capsules furnish the final product with an intangible value in terms of product identification. Perceived as an easy to swallow and more efficient dosage form, capsules improve adherence.
In terms of manufacturing, the reduced number of production stages leads to equipment, quality testing and operator savings, thus reducing the cost per unit.
In conclusion, when compared with tablets, hard capsules offer notable advantages throughout the drug product lifecycle (Figure 1), which increases the likelihood of both process and product success.
References
- J.E. Browne and D. Fulper, “Oral Dosage Forms Delivery Options for Lipid-Based Formulations,” Tablets & Capsules March/April 2021: pp 10–13.
- M.K. Biyani “Solving Formulation Problems with Liquid-Filled Hard Capsules,” Tablets & Capsules September 2018: https://tabletscapsules.com/wp-content/uploads/pdf/tc_20180901_0033.pdf.
- https://www.euroveg.eu/relevance.
- Capsugel, “Study of Consumer Preferences: Solid Oral Dosage Forms,” 2010: www.capsugel.com/knowledge-center/study-of-consumer-preferences-solid-oral-dosage-forms.
- Hermes Pharma, “A Hard Truth to Swallow?” 2015: www.swallowingtablets.com.
