It can be argued that the most important issue confronted by a pharmaceutical manufacturer is to ensure that products are not cross-contaminated. Should such an event occur, it will have an impact on the bottom line, as potentially expensive materials must be consigned to waste. Regulatory agencies will take action against the company if contamination issues are not fully investigated and corrected. Most critically, if undetected, patient safety is put in jeopardy.
In the contract manufacturing environment, where materials of widely differing pharmacological effects and potencies are handled, the need to ensure that cross-contamination does not occur is paramount. While it may be less glamorous than development of processes or formulations and less interesting than development and validation of key analytical methods, development of a robust cleaning programme is no less important. The cleaning programme will be an area of scrutiny for the contract manufacturer’s clients and will be extensively reviewed during the regulatory pre-approval inspection.
One of the challenges faced by the contractor is convincing new clients that cleaning method development and validation must be supported, and funded. Each client must trust that the contractor is treating cleaning with the same level of importance from client to client.
In addition, supporting documentation will be discussed. Careful evaluation of current cleaning procedures is essential, especially this year, as the FDA has announced that it will soon issue its first general draft guidance on cross-contamination.
develop a process
Before multi-use equipment is employed for a process, it is advisable to initiate development of the cleaning process. While management will be horrified if product is cross-contaminated, they often take an equally dim view of an expensive fixed asset lying fallow while someone tries to figure out how to clean it and how to demonstrate that it is clean.
When it comes to cleaning the equipment, Ash Stevens has found that the most effective, and reproducible, cleaning technique is to dissolve the active compound. Of course, this can pose challenges for novel compounds for which little literature exists.
The scientist developing the cleaning process should evaluate solubility in a variety of solvents, including water, acids, bases and alcohols. This rigorous exercise can often yield unexpected and desirable results. On several occasions, the company has learned that the compound was significantly more soluble in a different solvent, sometimes safer and less expensive, than initially believed.
When considering what should be removed from the equipment, the short answer is ‘everything’. Manufacturers must be sure that all active materials are removed from the equipment. Any catalysts must be removed and verified that they have been removed. Toxic solvents must also be removed. Finally, there should be no residual cleaning agents left behind. Even residual water can be harmful if the equipment will next be used for a synthesis that is poisoned by water. For certain applications, consideration needs to be given to microbiological contaminants.
Analytical techniques to be used for cleaning verification can include the following: Visual inspection under ‘white’ and UV light; Total Organic Carbon – useful for evaluation of water-soluble compounds (recognising that this method is not specific for the compounds of interest); UV spectroscopy; and, most frequently, chromatographic methods.
Analytical methods that will be used to evaluate cleaning samples must also be developed and suitably validated. Often, the same methods that are used to qualify the materials or the product can be adapted for verification of cleaning. It is important to confirm that the linear range and limit of detection/limit of quantitation are appropriate for the cleaning samples.
Swabs are often used to sample the cleaned equipment. Experiments should be performed to determine the amount of compound that can be transferred from the equipment to the swab and from the swab to the sample preparation for analysis. A known amount of sample can be placed on coupons, which simulate the surfaces to be cleaned. After swabbing the coupon, the tip of the swab is placed in the testing medium and sonicated. Finally, the ratio of compound detected by the analysis is divided by the amount placed on the coupon.
This percentage, called the “swab recovery” or “swab transfer efficiency”, should be as high as possible; references say greater than 70%. However, where some materials are found to be uncooperative lower numbers may be accepted.
qualified operators
Operators who will perform sampling of cleaned equipment should be trained and ‘qualified’. At Ash Stevens employees have to perform the ‘swab recovery’ experiment and evaluate them against the standard. They meet the requirement if they are within a reasonable amount of the ‘standard’ recovery.
The choice of swabs is important, too. Those designed for domestic use can contain perfumes or emollients that can interfere with the analysis so specialist swabs sold specifically for cleaning studies are recommended.
In designing a cleaning procedure, consideration needs to be given to the equipment to be cleaned.
During initial operational qualification (OQ) of the equipment, Ash Stevens would spend some time to locate ‘hard-to-clean’ areas. Often a water-soluble UV-active placebo is used to ‘dirty’ the equipment; the equipment is cleaned and inspected to evaluate any areas that still show evidence of the placebo. Photographs are useful to document the locations of the ‘hard-to-clean’ areas. Overheads, vent lines, vacuum lines and valves are notoriously difficult to clean and to prove that they are clean. Special attention will have to be given to these sites when cleaning and sampling.
clean process area
Consider also cleaning of the process area and how it will be executed and the cleaning verified. The materials and equipment to be used in the cleaning process must be specified. Companies are required to ‘qualify’ cleaning materials. Obviously, the analysis is not as involved as qualification for manufacturing, but at Ash Stevens we want to be sure that these materials are suitable and of consistent quality. For example:
- If 1N Hydrochloric Acid is specified and 0.1N HCl is provided, cleaning may not be as effective.
- Clean-in-place systems are preferable to manual cleaning because they take operator-to-operator variability out of play.
The next question is to consider what levels of residue can be tolerated in the equipment after cleaning. Toxicity data can be useful in calculating permissible residue limits. Often, however, these data have yet to be developed for novel compounds. Ash Stevens always takes a very conservative approach when determining permissible residue limits. In determining limits, references indicate that a 4-log reduction below toxic levels should be achieved.
Of course, all of this information must be documented. Typically, the company will prepare a ‘Cleaning Procedure Development Report’ to include all of these data.
Based on the cleaning development work, formal cleaning and analysis procedures are developed. Forms are prepared to document all cleaning activities: who is responsible for what; what equipment is to be cleaned; what materials are to be used; how cleaning is to be performed; how cleaned equipment is sampled; what are the analytical results; do the results conform to the permissible limits; and how is clean equipment secured, maintained and identified so it is ready for the next operation.
For its operation, Ash Stevens has prepared cleaning ‘batch’ records that detail all of the requirements and document which employee has completed them. One such ‘batch’ record is used to document each cleaning campaign. After all of the cleaning and analysis is complete, the record is reviewed by the Lead Operator and by Quality Assurance. If all of the documentation is in order and the analytical verification to the criteria, QA will sign off that the equipment is clean.
All activities discussed up to this point serve to verify that equipment has been suitably cleaned. It is expected that the cleaning process be formally validated, as well.
Just as with Process Validation, Cleaning Validation requires a pre-approved protocol that details who will be responsible for what, procedures to be followed, the number of cleaning cycles that will be evaluated, the time frame for validation and the acceptance criteria. Any special conditions, procedures or sampling should be discussed. The protocol should indicate that a summary report is to be written once the validation procedures are completed.
One thing to consider during formal Cleaning Validation is that ‘dirty’ hold times should be evaluated. That is, ‘what is the acceptable duration between the completion of the process and the initiation of cleaning?’ The concern is that it is often more difficult to clean something that has been dirty for a long time.
During Cleaning Validation, the company will also take ‘baseline’ rinse and swab samples of the equipment before it has been cleaned. Most often, these samples are loaded with compound. Rinses and swabs taken after cleaning show no residue. This is a very clear demonstration that the cleaning process is doing what it is supposed to. Also, extra samples of the cleaned equipment may be taken, again to demonstrate clearly that the cleaning process is effective.
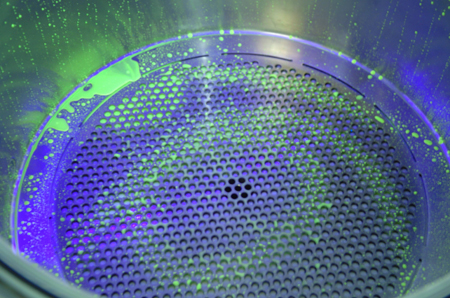
Ash Stevens uses photography to help document hard-to-clean areas while designing cleaning procedures. Above: A UV-active placebo highlights residues before cleaning. Below: After washing procedures, the agent is well removed

We will typically plan to execute Cleaning Validation concurrent with Process Validation. This can have an advantage in that the prescribed number of cleaning replicates and analyses can be conducted more efficiently, particularly in the analytical laboratory.
After the prescribed number of replicates, a Cleaning Validation report is prepared. The report summarises all of the cleaning activities. Were there deviations in the cleaning process? Were limits consistently met? If ‘re-cleaning’ was required, the overall validity of the cleaning process should be questioned. Summarise the analytical data.
If there are procedural changes that are indicated by this controlled study, they should be discussed. Often, many samples will exhibit no detectable residue. Consideration can be given to removing these samples during future routine cleaning verification campaigns. ‘Baseline’ samples are not required after validation. All changes to the cleaning process should be managed under change control.
The report should conclude with a statement as to the validity of the process. Key members of your organisation, and often your client, must endorse the report.
Some interpret ‘validation’ to mean that the analysis is no longer necessary once the cleaning process is validated. The ramifications of cross-contamination are so significant that manufacturers should assess whether this would be worth the risk. Ash Stevens always performs analytical verification of multi-use equipment before it can be used for another product.
Demonstrating that equipment is free of potential contaminants that could affect the quality of a customer’s product is an essential element of pharmaceutical operations.
Bibliography:
G. Bismuth and S. Neumann 2000 Cleaning Validation: A Practical Approach, CRC Press LLC, Florida, US

