Dr Kevin Robinson (KSR): By launching PharmaControl, PharmaCheck and PharmaResearch, Korsch was a front runner in the race to upgrade and stabilise the tableting process by adding digital features. What was the rationale behind the development of these systems?
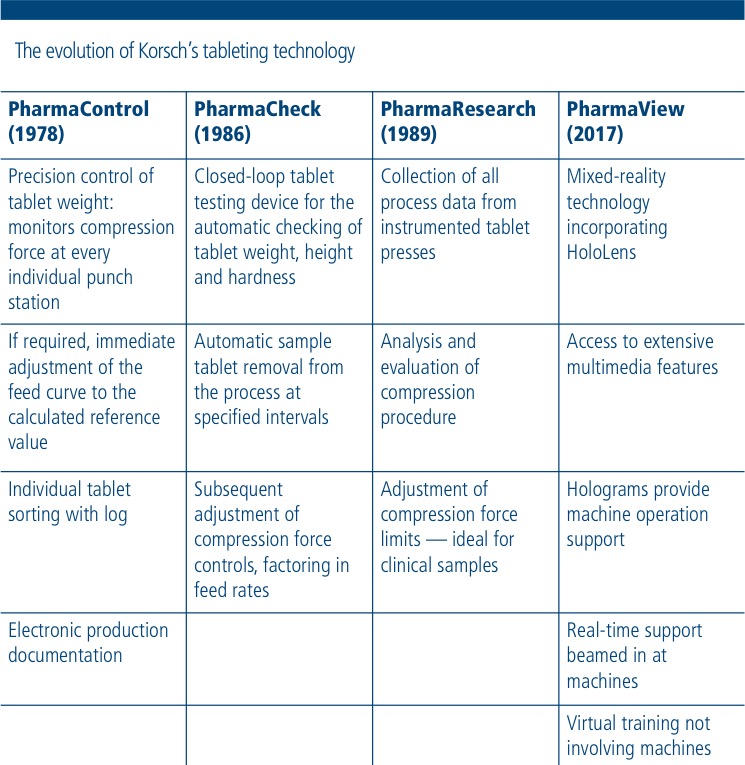
Stephan Mies (SM): If you take a closer chronological look at our product range, one thing strikes you immediately — how early we began to focus on digitisation. The term itself came later, but PharmaControl originated back in the 1970s. That was the first step on the road to designing our products to be smarter and furnishing our products with additional benefits, which makes machine operation routines more convenient and reliable.
Arno Rathmann (AR): Pharmaceutical product features have always been synonymous with digital, networked solutions that address everyday tableting problems. These solutions measure, monitor, control and analyse — in short, they collect data that we can channel into our systems as required for process improvement purposes.

KSR: So, your path to digitisation was paved by automating the tableting process?
SM: That certainly played a major role. Korsch was an automation pioneer in the 1990s. We accepted the fact that the market and system environments had not got that far yet … and virtually took the plunge because we were then — and are still now — firmly convinced that we are on the right track.
The various stages of evolution that the XL 4004, now in its fourth generation and equipped with smart components, has gone through demonstrate this development process really well.
KSR:What does “digitisation” mean to Korsch nowadays?
SM: Two years ago, as part of our annual strategy meeting, we took a conscious decision to focus on digitisation. In my opinion, anyone that isn’t receptive to this new perspective on technology doesn’t have a viable future. That’s because we are increasingly observing how our customers are becoming more and more responsive to this concept. And yet the pharmaceutical industry in general tends to be regarded as a conservative sector of the economy. The additional benefits just need to be explained to those that haven’t taken the same leap of faith.
AR: Exactly. Digital products appeal to users if they take account of three issues: security, productivity and service. We don’t do digitisation and technological enhancement for the good of our health, but to help provide our customers with better quality, cost-efficiency and convenience during day-to-day machine operations. Digitisation is the so-called “enabler” in this process — enhancing continuous communication between both machines and operators.
SM: In that respect, nothing has basically changed at Korsch. We create optimum solutions for users based on the dialogue we have with customers, always in line with the aspiration of offering more, because we are the specialists. That’s why we have a dedicated employee whose remit is digitisation. This person analyses processes, both our own and those of our customers, evaluates them, creates concepts and actions them together with the engineers.
KSR:To what extent have customer requirements changed with time?
SM: Data flow is increasingly becoming a key issue. Data security is one of the most critical factors in terms of customer acceptance and, ultimately, the success of digitised products. That’s because pharmaceutical production data is generally highly sensitive. Therefore, it is vital to provide our clients with in-depth advice on this issue and create specific security solutions together with them.
AR: As well as prioritising data security, it’s also important to harmonise programs, systems and user interfaces. That’s the only way that interface communication within a continuous process such as tableting can be ensured. For example, we are working hard on this issue together with our partner, L.B. Bohle.
KSR: Digitisation isn’t just for tableting machines and systems, of course; can you say a few words about PharmaView for operating and service processes?
SM: The use of virtual technologies enables us to provide a much faster, more complex service. Our solution is based on mixed-reality technology incorporating Microsoft HoloLens.
AR: The operator is provided with a large quantity of practical ancillary information and functions on an augmented reality basis, enabling them to better understand how the machine works. Day-to-day processes as well as maintenance or repairs are thus simpler and faster. The first pilot projects have already been initiated.
KSR: Are there any other future-oriented topics that you are addressing within Korsch?
SM: Digitisation always occurs on two levels: products for our customers and within our own company where the objective is to improve, accelerate and create more efficient processes. As part of this digitisation trend, the issue of “knowledge management” has taken on enormous importance for every company. Big Data is one of the major challenges of the 21st century. For us, that means gathering enormous quantities of data in a meaningful and sustainable manner, giving them transparency, making them available and, of course, archiving them securely for the long-term.
KSR: And how does that work in practice?
SM: Korsch is a company with sustainable, long-term employee relationships. We have always benefitted from them. Our team, like many others, has been undergoing a generational change or a “changing of the guard” if you like for several years now. That brings in new ideas and the power of innovation … but also involves the risk of knowledge loss. Experience and know-how must be sustainably entrenched and preserved within the company; otherwise, we will regress instead of progress. The digitisation of processes is a very fundamental aspect of proprietary knowledge management.
AR: We only need to take a look at how our information absorption habits have changed during the last few decades. People very rarely leaf through user manuals these days; we tend to Google the problem or watch a tutorial on YouTube. We must also take these new preferences seriously in the workplace environment.
SM: That’s why our task is to digitally record and cross-departmentally utilise all the information that still flows from employee to employee in analogue mode. That plays a major role, for example, in production process communication between the Product Development department and Assembly Operations.
KSR: And finally, gazing into the crystal ball, what role might “Innovation Made in Germany” play in the evolution of Industry 4.0?
AR: Of course, a lot depends on what foundations are laid in terms of infrastructure and specialist human resources in the next few years. Germany as a research, development and production location continues to represent a seal of quality.
SM: The technology advantage that we derive from our R&D activities enables us to serve a specific market segment, in which growth is just as innovation-driven as in ours. At the same time, we want to encourage our customers to join us on this new path into the digital era by engaging with us on joint projects. Then, both sides will benefit from the power of innovation in Germany and create products — which are often better than “standard” offerings — that are in demand in the global market.
The original version of this article appeared in KORSCH:MAGAZINE 1, 4–6 (2018) and is reproduced with kind permission.
