Faster development times are required to progress biologics and vaccines more rapidly into clinical development and then to market to improve worldwide accessibility.
Twinned with this is the need to reduce the COGs of biologics by 90%, says industry body Biophorum Operations Group (BPOG), to make them affordable for growing numbers of people globally.1
These two drivers have led to the requirement of greater flexibility when designing manufacturing facilities that can accommodate a growing range of different types of biologics and can be reconfigured easily to accommodate the high attrition rates of monoclonal antibody (mAb) or gene therapies in clinical trials (only one in 10 mAbs and even fewer gene therapies will be successfully licenced for patient use).
To meet these challenges, the biopharmaceutical industry is adopting strategies such as intensified and continuous processing that regulatory bodies such as the US FDA are endorsing as they believe this will help to achieve higher product quality while making new therapies more affordable.
For intensified and continuous bioprocessing to deliver on these quality and productivity promises, there is a requirement for intelligently designed products; Sartorius Stedim Biotech (SSB) has, in partnership with the biopharmaceutical industry, developed a toolbox of technologies that are discussed in detail in this article.
Overview
Currently, the biopharmaceutical industry is concentrating on three areas in which upstream bioprocessing can be intensified. The first is in the seed train; here, intensification can be achieved by directly inoculating cells from high cell density cell banks into rocking motion (RM) bioreactors and is a strategy that biopharmaceutical companies are implementing as it eliminates the need to use shake flasks in the post-thaw expansion phase.
Also in the seed train, perfusion approaches — whereby cells stay in the exponential growth phase throughout the entire culture — are being used to skip steps or generate higher cell densities to inoculate the production bioreactor.
For example, an N-3 culture in a RM50 bioreactor (5–25 L working volume) can be used to directly seed a 500 L bioreactor to skip the N-2, 200 L bioreactor stage; or, an N-1 500 L culture in a stirred or RM bioreactor can be used to seed a production culture with a final volume of 2000 L at a high inoculation density. These different seed train intensification approaches are shown in Figure 1.
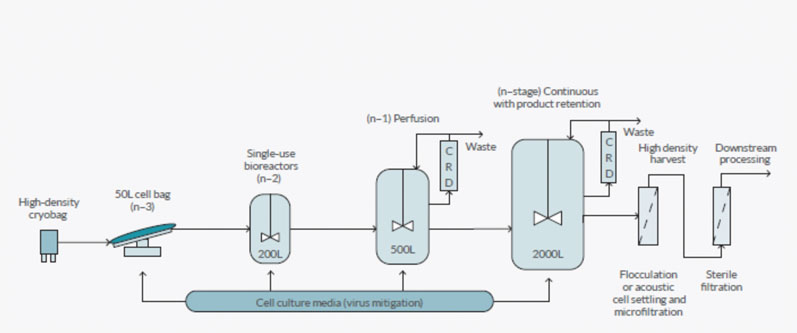
Figure 1: An overview of the different approaches to process intensification
Intensification approaches can also be used in the production bioreactor. A hybrid approach, whereby the seed train is run in perfusion mode with an N-1 500 L culture being used to seed the main bioreactor (2000 L) with a high cell density, which is then run in fed batch mode to purify product from the bioreactor, is a platform approach being used by companies including Bristol-Myers Squibb.
Other companies have installed or are developing platforms to run their production bioreactor in perfusion mode and either retain the product in the bioreactor for batch harvesting (Amgen) or purify their product from the permeate (Pfizer and WuXi AppTec).
However, process intensification can present a problem if and when cell densities in the bioreactor become so high that traditional separation technologies cannot remove all the cells and cellular debris. As a result, different types of separation technologies may be required to separate or harvest the cells from the permeate using a retention system.
Cell lines, media platform and cell banking
For process intensification in the seed train or main bioreactor, cell lines that can be cultured in perfusion conditions are required and SSB offers the Cellca CHO Expression Platform for this application.
To demonstrate the performance of these cells under perfusion conditions, Cellca CHO cells expressing a model mAb (IgG1) antibody were cultured in fed batch and simulated perfusion conditions with a shaker-intensified batch simulation protocol using daily centrifugation and medium replacement with standard Sartorius cell culture media and feed mix (1 volume/day perfusion for 15 days).
Using the fed-batch culture conditions, 25 x 106 cells were produced, whereas a higher cell density of 30 x 106 cells was achieved using the simulated perfusion conditions. This cell density was deliberately restricted by a temperature drop-induced cell growth arrest (Figure 2).
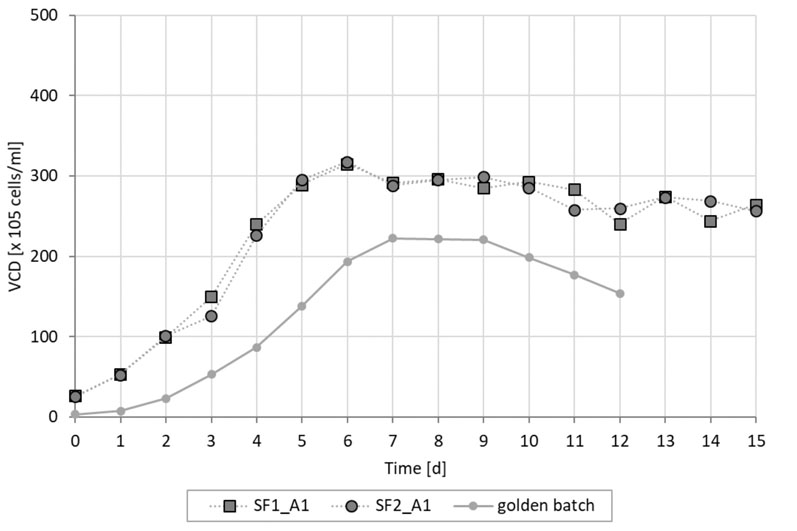
Figure 2: Viable cell density of Cellca CHO cells cultured in fed batch and intensified batch conditions (SF = simulated perfusion in shake flasks; golden batch is average fed batch trajectory)
Although the cell density achieved was not significantly higher, the titres were (Figure 3). With the fed-batch conditions, 3 g/L was produced, whereas the Cellca CHO cells cultured using the simulated perfusion conditions produced a three-fold greater titre in 12 days and a four-fold titre increase to 12–14 g/L in 15 days.2
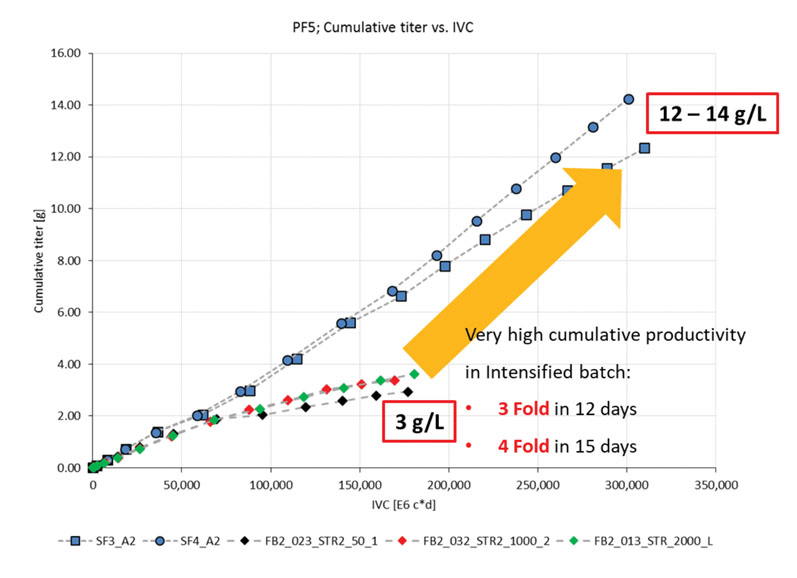
Figure 3: Titre produced from Cellca cell line cultured in fed batch and intensified batch conditions (SF = simulated perfusion in shake flasks; FB = fed batch at different stirred tank reactor scales)
SSB also offers a cell bank manufacturing service, which can save time with intensified processing as the high cell density vials produced can be used to directly seed RM bioreactors. The cell banking service, housed in a 260sqm cleanroom space, is a Medicines and Healthcare products Regulatory Agency (MHRA)-accredited manufacturing facility holding an Investigational Medicinal Products (MIA, IMP) licence.
The facility uses media qualified in process simulation trials and an automatic closed single-use vial filling system, Fill-It, which can fill 500 vials with up to 5 mL at 50 x106 viable cells/mL and reduces the risk of contamination.
The cell banks are characterised in accordance with ICH guidelines in a good manufacturing practice (GMP)-accredited testing facility and are QP Released within five months of vial initiation. SSB is also currently working on methods of freezing high cell densities in bags as an additional seed train intensification option.
References
- www.biophorum.com/wp-content/uploads/2017/11/Biomanufacturing-Technology-Roadmap.pdf.
- D. Mueller, et al., “A Versatile Toolbox for Rapid Development of Intensified CHO Processes,” poster 554 presented at ESACT 2019 (www.esact2019.com/wp-content/uploads/2019/05/ESACT-2019-Poster-Abstracts.pdf).
Part II of this article will appear in the September 2019 issue of Manufacturing Chemist.
