Mycoplasma can be a costly safety hazard for the biomanufacturing community. Martha Rook, Frank Panofen and Wayne Miller at Millipore Corporation a new rapid test that can help companies reduce the financial risk and optimise product yields.
Mycoplasma contamination in biomanufacturing facilities is a serious concern for the safety, purity and potency of drugs. It can have costly consequences for biopharmaceutical manufacturers, who must destroy an entire batch and shut down production facilities for an extended period when mycoplasma contamination is detected.
Mycoplasma are small, slow-growing bacteria of the class Mollicutes. Other Mollicutes, including Acholeplasma and Spiroplasma, are also a contamination concern; however, mycoplasma has become the generic name assigned to micro-organisms in this class.
These organisms lack a cell wall and are therefore resistant to antibiotics that target cell wall synthesis. Several mycoplasma are disease-causing in humans, including those linked to atypical pneumonia and other respiratory disorders, and another believed to be a cause of pelvic inflammatory diseases. There is also some evidence that they may cause or contribute to some cancers.1
Mycoplasma contamination can be a significant challenge for biopharmaceutical manufacturing. Cell cultures are vulnerable to contamination from both contaminated raw materials and operators themselves. As Mollicutes are known to infect a wide variety of eukaryotic hosts, including mammals, birds, reptiles, fish, insects, and even plants, they could enter a production process through virtually any raw material. Contamination from operators is also a concern as humans are often carriers of organisms such as M. orale and M. salivarium.
Detecting and eliminating mycoplasma from biopharmaceutical manufacturing processes is extremely difficult due to their size – less than 1µm. Mycoplasma is difficult to detect in cell cultures as it generally does not lead to visual turbidity in media or pH changes. However, infection can affect host cell metabolism and expression levels and ultimately lead to the loss of cell lines. The small size of mycoplasma and its flexibility due to the lack of a cell wall also means it can pass through the normal 0.2µm sterilising-grade filters that biomanufacturers use to protect their cell culture when they filter media to remove any contaminants.
Traditional growth-based methods to test for mycoplasma contamination take as long as 28 days, which poses an extreme problem for biomanufacturers. If they have completed an entire manufacturing process and have the product ready to be shipped to market when they find that they have experienced a contamination event, the batch would have to be scrapped. In addition to that, these slow methods do not allow for an appropriate investigation and therefore the root cause analysis for a contamination is very difficult and time-consuming.

The dual membrane filtration device
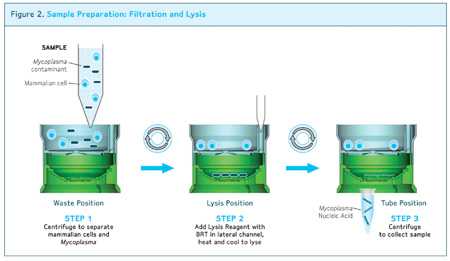
Samples are processed in a simple three-step procedure (below) using a dual membrane filtration device
Since the root cause of mycoplasma contaminations is rarely identified, it is important to implement a comprehensive programme within the biotechnology manufacturing process to prevent, reduce and detect mycoplasma contamination. The ability rapidly to detect a contamination event in raw materials or early in a manufacturing process is a key to any risk reduction programme.
Therapeutic products for both human and animal use are required to undergo mycoplasma testing throughout various steps in the production process including: cell lines/banks, virus seeds, fermented samples, crude harvests, bulk vaccines and purified biological products for human use.
The compendial mycoplasma test method includes both a culture and an indicator cell test. The culture test is performed by inoculating the sample in both broth and agar media and incubating at two different temperatures. The cultures are observed and sub-passaged several times over the course of 28–35 days. If no contamination is found within this timeframe the sample is considered negative.
As not all mycoplasma is capable of being cultured using specialised mycoplasma media, an indicator test is also required. In the indicator test, the sample is inoculated in indicator cell lines and incubated over the course of 7–10 days. At various points in the incubation, the cultures are stained with a DNA binding fluorescent dye and the cultures are examined microscopically for the presence of extra-nuclear fluorescence that indicates the presence of mycoplasma.
These tests are generally performed following guidelines in the European Pharmacopeia chapter 2.6.7 “Mycoplasmas”, 21 CFR 610.30 Code of Federal Regulations 221 CFR Ch. 1 Subpart D Mycoplasma, and the Center for Biologics Evaluation and Research, FDA Points to Consider in the Evaluation of Cell Lines Used to Produce Biologicals 1993.
Due to increasing concerns with the impact on both drug safety and supply and the financial impact of a mycoplasma contamination event, there has been an increased trend towards in-process screening for mycoplasma by biopharmaceutical manufacturers.
This screening has benefited from the introduction of improved nucleic acid techniques (NAT) specifically designed to meet the requirements of a rapid mycoplasma detection system for biopharmaceutical use.
Most NAT methods are based on the amplification of the nucleic acid content of a mycoplasma cell by enzymes. Methods such as polymerase chain reaction (PCR), real-time transcription mediated amplification (Real-Time TMA; Gen-Probe Inc) and microarray-based detection methods are currently available.
These amplification methods are sensitive enough theoretically to detect the nucleic acid content of a single cell. Specific nucleic acid sequences are targeted for amplification and can be used to ensure that only the organism of interest is amplified and detected.
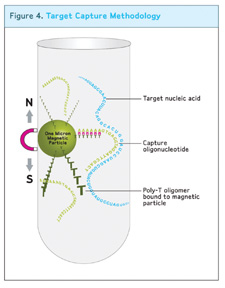
Target capture methodology: Target rRNA present is captured on the magnetic beads and cell debris, proteins, and non-specific DNA and RNA are washed away from the target
For a method that requires multiple species to be detected, a common nucleic acid target sequence is ribosomal nucleic acid. Ribosomal nucleic acid makes an ideal target because it is highly conserved among species so a single assay can detect multiple mycoplasma targets. Assays can further target either ribosomal RNA (rRNA) or DNA (rDNA).
Targeting rRNA has the advantage that rRNA is present in hundreds to thousands of copies per cell while rDNA is present in 1–2 copies per cell, potentially increasing the sensitivity of an rRNA-based assay. Additionally, rRNA is less stable than rDNA and therefore more likely to be an indicator of the presence of viable mycoplasma contamination.
One of the drawbacks of NAT methods is the generally limited sample volume that can be input into these methods. While the compendial growth method uses 10mL of test article, most amplification methods are limited to 1mL or less starting volume, which makes comparison of sensitivity with the traditional method difficult. Additionally, these methods are often vulnerable to both nucleic acid and cellular contamination that can lead to false positive results. Generally, strict workflow requirements and a high degree of training in molecular biology training are required to run NAT methods efficiently.
One of the newest developments in the field of RNA detection is Millipore’s MilliPROBE Real-Time Detection System for Mycoplasma, which provides mycoplasma presence/absence results within four hours.
The MilliPROBE assay starts with Millipore’s sample preparation technology to allow very representative sampling of up to 20mL of test article. The sample preparation is then coupled with Millipore collaborator Gen-Probe’s Target Capture and Real-Time TMA technology using Gen-Probe’s newly developed technology called Background Reduction Technology (BRT).
BRT protects the assay from false positive results by ensuring that contaminating nucleic acid or mycoplasma cells that enter the assay after the closed sample preparation step cannot be amplified or detected. The combined technologies of a closed, high-volume sample preparation and the BRT lead to a very robust assay with very low false positive rates and allow the assay to be run without the workflow controls used with typical NAT tests.
Since mycoplasma testing is highly regulated, new tests, such as this nucleic acid amplification method, must first be shown to be comparable to the general growth-based method typically used in the US. This standard requires the user to demonstrate that the test can detect <10 colony forming units (CFUs)/mL of mycoplasma without detecting non-mycoplasma contaminants.
The MilliPROBE system detects <1-10 CFU/mL for a panel of mycoplasmas and has been demonstrated not to detect a panel of other common bioreactor contaminants.
In addition, the technology complies with recently updated European Pharmacopoeia regulations that contain specific guidelines for how to validate a nucleic acid test for the detection of mycoplasma.
Millipore supports the new system implementation in a variety of ways. The company’s applications group can evaluate the particular cell matrix to ensure that it works well with the sample preparation system and amplification system. On-site validation services are available to validate the instrument platform and bring it up on the customer site.
In those situations in which a particular biomanufacturer is not permitted to use live mycoplasma in its laboratories (usually when a quality control laboratory is at the same site as the manufacturing facility), Millipore’s MicroSafe BioSafety Solutions Laboratory, Europe can establish a testing protocol and carry it out to demonstrate that the assay can detect live mycoplasma.
Reference:
1. www.molecularstation.com/cell/Mycoplasma-contamination/, Retrieved April 23, 2010.
