Expanding manufacturing capacity and taking advantage of new technologies
Designing and building new pharmaceutical processes is a complex and expensive task. New processes or skids can be simplified and made more cost-effective by improving communication and working with an experienced solutions provider. By coordinating the different design groups and optimising the purchasing process, new projects can be delivered with minimal footprints and enhanced performance.
Once the overall concept for a project has been agreed, the process of designing the physical structure begins. Attention also turns to approved suppliers of components and how these will be used to deliver the finished process. Often, this follows a well-trodden path of engaging certain suppliers for specific products, but there is an opportunity to exploit other products from the same suppliers, reducing project complexity.
From this point, the process of designing and installing a new pharmaceutical process usually involves three main groups: mechanical engineering dealing with pipework, vessels and valves; electrical and instrumentation engineers taking care of valve control and electrical installation; and the automation engineers responsible for the control concept and implementation.
Each group has their own set of goals that, together with designers and contractors, will deliver the project for the end user. However, these groups will often act independently once the overall concept for the process has been decided. They will proceed with designing and specifying the components they need from approved vendors to deliver their section of the project.
Broadening the view
For the mechanical section, designers will often use products and layouts from previous projects. For the design stage, this means that they already have the 3D models to use in the ‘virtual layout’, allowing the majority of the design to be delivered relatively quickly.
However, this can mean that more compact designs are ignored or improved functionality is not implemented, even when enhanced components are available and meet all of the build criteria. By taking consideration of a wider range of equipment and addressing potential challenges early in the design phase, many benefits can be achieved.
As a long-established manufacturer of control valves and a designer of flow control systems, Bürkert can offer experienced insight that is supported by cutting-edge technology. Working in partnership with the process designers, it is possible to identify solutions that might otherwise be overlooked. Furthermore, with considerable experience in designing products for the pharmaceutical sector, Bürkert is well-placed to ensure compliance with industry standards.
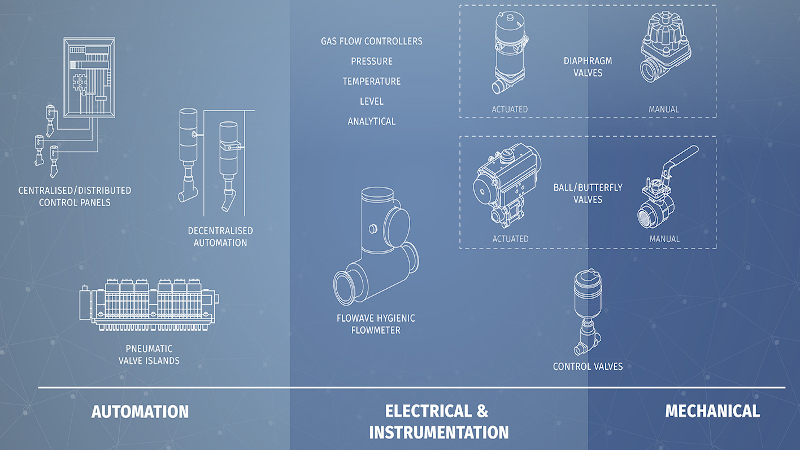
Improving designs
One of the criteria often raised for a project is to reduce the number of welded joints and minimise the dead space in the system. Bürkert is able to use its design expertise and manufacture a number of specialty distribution valve blocks that achieve this aim while matching all other hygiene standards applied to the rest of the installation.
By working across the three major design groups, Bürkert can deliver a more integrated solution. By working in partnership with OEMs, it is possible to take advantage of additional cost savings as well as reduce the overall time for installation and commissioning.
Expertise on hand
Bürkert’s Hygiene Competency Centre (HCC), offers a central pool of expertise that can be targeted at a specific issue or challenge. As the project design progresses, there will often come a point where a number of valves or actuators appear not to fit in the space available. In many cases this can be achieved using a bespoke valve manifold or alternative valve configuration. Taking expert advice on what can be achieved using the latest manufacturing techniques or valve designs can often resolve these issues.
HCC contains the resources to allocate engineers to work with the original project team and provide assistance to all three areas of the design. A local point of contact or project manager is assigned responsibility for coordinating the communication and ensuring all aspects of the project are covered.
By taking a more holistic approach, providing project management support, design expertise, documentation, step files, drawings, 3D models, simulations and sizing tools for control valves, the whole project can become more efficient. Working in partnership with an expert in pharmaceutical design can achieve savings in overall project costs, but more importantly, improved efficiency and process reliability.

