In this article, Gary Watts, Senior Manager, Analytics, at Abzena, delves into the nuances of the formulation process, shedding light on its critical importance in the journey from lab to market.
Formulation development is more than just creating a drug that works, it helps to ensure that the drug reaches those who need it most in a form that maximises its therapeutic potential.
The role of formulation
The formulation process starts with an evaluation of the drug's physical and chemical properties to understand its solubility and stability. Poorly soluble drugs often lead to reduced bioavailability, thus rendering them ineffective.
Techniques such as particle size reduction, salt formation and the use of solubilising excipients are employed to enhance dissolution. Stability, by contrast, is crucial to maintain the drug’s efficacy with time. In this case, techniques such as lyophilisation and the use of stabilising agents help to preserve the integrity of the drug under various conditions.
Maintaining stability in different levels of acidity is essential, especially if you consider drugs that pass through varied pH environments in the body. Buffer systems, enteric coatings and pH modifiers are used to ensure stability.
Additionally, enhancing bioavailability, the degree and rate at which a drug is absorbed, is paramount. Methods such as lipid-based formulations and nanoemulsions have become increasingly popular to improve bioavailability, especially for drugs with poor aqueous solubility.
Formulation scientists also have to address the delivery method of the drug. Whether it’s oral, injectable, topical or inhalable, each form has its own set of challenges and considerations.
For instance, creating a drug that is easily ingestible and palatable, or one that can be effectively absorbed through the skin, requires distinct approaches and technologies.
Whole industries have evolved that solely develop formulations for ease of use — such as effervescent tablets or orally disintegrating granules (ODGs) — in the quest to improve adherence.
Moreover, in today’s pharmaceutical industry, in which personalised medicine is becoming more prevalent, formulation must also cater to specific patient needs. This might involve developing formulations that are suitable for different age groups or for patients with specific health conditions.
In essence, formulation is where science meets practicality. It’s about ensuring that the drug does what it’s supposed to do in the most effective and safest way possible.
It’s a critical aspect of drug development that requires a thorough understanding of the drug’s properties and the body’s responses.
A proactive approach
When it comes to a new formulation project, it’s important to prioritise the early detection of potential drug instabilities.
At Abzena, we take a proactive approach to formulation development that involves conducting stress tests at the preliminary stages, which helps to identify issues before they escalate into major obstacles.
By recognising and addressing these challenges early, you can help clients to avoid costly delays and setbacks that could occur during later phases and/or clinical trials.
A best-practice formulation strategy is a methodical process that encompasses several critical stages to ensure the efficacy and safety of drug compounds.
Stage one: manufacturing stresses and analytics
The process begins with assessing the drug’s response to different pH levels and includes techniques such as low and high pH stress tests.
Analytical methods including size-exclusion high-performance liquid chromatography (SE-HPLC) and capillary isoelectric focusing (cIEF) provide insights into the drug’s molecular characteristics. This allows you to predict and mitigate potential issues related to solubility and stability.
Stage two: preformulation
In the preformulation stage, we use thermal ramp studies to investigate the impact of factors such as pH and charge on the formulation, providing early indicators for necessary adjustments.
This stage acts as a bridge between initial assessments and more advanced stages, ensuring that the formulation is on the right track.
Stage three: Premanufacturing optimisation
Alignment with process development (PD) and manufacturing is critical at this stage. It includes assessing the impact of concentration on tangential flow filtration (TFF) and determining the need for surfactants using agitation studies.
Advanced analytics such as viscosity and osmolality measurements are incorporated alongside SE-HPLC and dynamic light scattering (DLS).
Stage four: extended stability testing
Extended stability testing forms the next crucial stage; this aims to assess the drug’s stability during a period typically aligning with the duration of Phase I clinical trials.
It includes a range of analytics such as capillary electrophoresis (CE-SDS) and determination of the drug-antibody ratio (DAR), alongside exposure to accelerated stability conditions (elevated temperatures and intensified light exposure) that provide vital early insights into the drug’s behaviour under less-than-ideal storage or environmental conditions.
Stage five: freeze-thaw challenge
This stage tests the formulation’s resilience to freeze-thaw cycles, which is particularly important for formulations that are stored as frozen liquids.
Technology transfer
The culmination of these stages is technology transfer, during which all data and insights are shared collaboratively with customers and the process development and manufacturing teams.
This ensures that a lead formulation is selected for further development stages, derisking more expensive stages of development. Through this co-ordinated combination of scientific rigour and meticulous planning, detailed formulation efforts contribute significantly to drug development successes.
This best-practice process makes sure that drugs not only reach patients with minimal delay but also ensures that they’re effective, safe, stable and optimised.
Case studies from Abzena
To better understand formulation in practice, let’s examine some specific cases.
Case study one: developability and strategic selection
We evaluated two potential drug candidates under a series of stress tests to assess their manufacturability, including acidic, basic/deamidation, agitation, freeze-thaw and oxidation conditions.
Whereas both candidates showed similar resilience against agitation, freeze-thaw, and oxidation, they diverged significantly under acidic and deamidation stresses.
Candidate 1 showed a notable decrease in monomer percentage and an increase in acidic species under forced deamidation, indicating a higher level of degradation compared with candidate 2 (Figure 1).
Additionally, candidate 1 exhibited a significant reduction in monomer percentage after just 2 hours of acidic stress, suggesting that low pH viral inactivation methods might be unsuitable for it.
These findings led to a strategic decision to discontinue the development of candidate 1 and focus resources on candidate 2, which showed more promising results.
This candidate then progressed to a full formulation study and was scaled up for subsequent toxicology and Phase I clinical trials. Our developability study accounted for less than 1% of the total manufacturing costs, underscoring the value of thorough early stage testing in drug development.
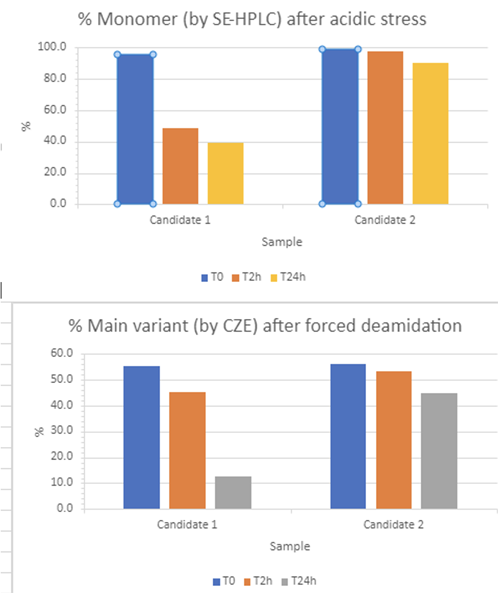
Figure 1: Developability and strategic selection; acidic stress and forced deamination results
Case study 2: creating a dual-purpose drug formulation
This project saw us develop a frozen drug product for intravenous (IV) delivery during a Phase I trial with a parallel focus on a formulation suitable for 2–8 °C storage and potential subcutaneous (SC) delivery post-Phase I.
After extensive formulation and a 26-week stability study, the lead formulation was chosen for its stability, lower viscosity (crucial for SC injection) and approved excipients. Stability tests at 2–8 °C and –80 °C showed similar results for high-concentration products (Figure 2).
This dual-purpose formulation approach enabled a faster transition to clinical trials and provided early insights into long-term stability and shelf-life, all for less than 2% of the total manufacturing costs.
This early investment in dual product formats not only expedited the formulation’s readiness for clinical trials but also provided valuable insights into its long-term stability and feasibility.
This case study showcases the strategic and flexible approach to pharmaceutical development, balancing immediate clinical needs with long-term commercial and therapeutic possibilities.
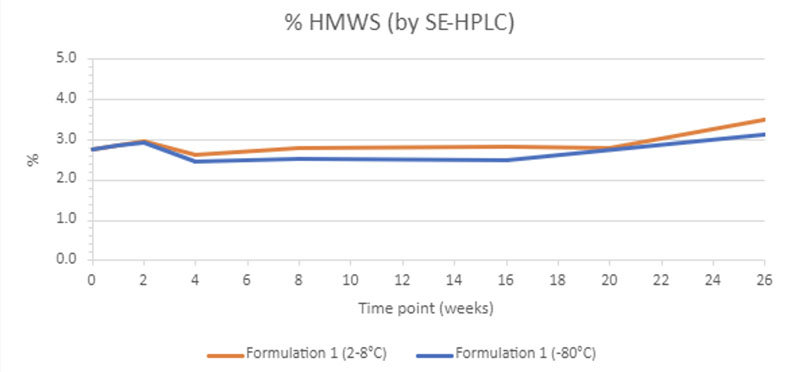
Figure 2: Creating a dual-purpose drug formulation; per cent aggregates for the subcutaneous product during the course of the study at both 2–8 °C and –80 °C
Industry wide benefits of formulation development
Formulation has obvious benefits for those drug developers looking to create a new product. But formulation, when properly implemented, has benefits that extend across the industry.
Enhanced drug stability and shelf-life
Advancements in drug formulation significantly enhance drug stability, leading to extended shelf-life. This has a cascade effect on the pharmaceutical industry, notably in terms of logistics and inventory management.
A longer shelf-life reduces the need for frequent manufacturing cycles, which can be both cost-effective and environmentally beneficial.
Additionally, it ensures that patients have access to effective medications for longer periods, which is crucial in regions with limited access to regular pharmaceutical supplies.
Broadens patient reach and ensures compliance
Effective formulation also improves the accessibility of drugs. By addressing issues such as solubility and palatability, medications become easier to administer, significantly impacting patient adherence to treatment regimens.
This is particularly important in chronic diseases when long-term medication adherence is critical. Improved formulations can also lead to the development of alternative drug delivery systems, such as transdermal patches or inhalers, offering more options for patients who may have difficulties with traditional delivery methods.
Finally, proper formulation can mitigate cold chain challenges by enhancing a drug’s stability at a range of temperatures, reducing its reliance on strictly controlled storage conditions.
Optimises therapeutic outcomes
The end goal of drug formulation remains to optimise therapeutic outcomes. A well-formulated drug ensures that the active ingredients are delivered in the most effective manner, maximising the potential benefits to the patient.
This not only translates to better health outcomes but also contributes to the overall efficiency of healthcare systems by reducing the need for additional treatments or hospitalisations owing to ineffective medication.
Reduces costs and time to market
Effective formulation strategies significantly streamline the drug development process, leading to reduced costs and faster market entry. By identifying and addressing formulation challenges early, you can help to prevent costly late-stage failures.
This efficiency not only benefits pharmaceutical companies in terms of reduced development costs, but also accelerates the availability of new treatments to patients.
Drives innovation in medicine
The continuous advancement in drug formulation techniques fosters innovation in medicine. It allows researchers and developers to explore new therapeutic areas, experiment with novel delivery systems and, ultimately, contribute to the advancement of medical science.
Formulation is not just about overcoming present challenges, it’s also a platform for future innovation.
Conclusion
To sum up, the role of formulation development is a critical step in the drug optimisation process. Formulation ensures that drugs are not only effective and safe but also accessible and cost-effective.
By taking a proactive approach to formulation development, you can identify issues and address challenges earlier in the development process that can reduce costs, minimise risks and improve the likelihood of downstream success in clinical trials.
The implications of advanced formulation techniques extend beyond individual drugs to influence a significant number of important areas. As advancements in this field continue, our ability to generate even more safe, stable and effective therapies will help to move medicine forward.

