Touchlight has announced the expansion of its groundbreaking mbDNA platform with the introduction of three new circular DNA architectures: sscDNA, hsscDNA and dscDNA.
These innovative constructs join mbDNA, which debuted earlier this year, to form a comprehensive portfolio designed to overcome the limitations of traditional gene editing tools.
The mbDNA platform and new custom circle DNA architectures deliver enhanced stability, reduced immunogenicity and compatibility with a broad range of gene insertion and expression technologies.
This sets a new standard for precision and performance in genetic engineering.
mbDNA (megabulb DNA) is a pioneering single-stranded DNA technology produced through Touchlight’s cell-free enzymatic platform, delivering high purity and minimal immunogenicity for safer outcomes, plus enzymatic production for optimal reliability and scalability.
It achieves high HDR efficiency with low toxicity, consistently reaching knock-in rates of 60-75% in primary T cells, while offering predictable performance with low donor-donor variability.
Additionally, mbDNA supports expanded payload capacity of up to 20kb, enabling the delivery of large genetic cargos and unlocking new possibilities for advanced gene editing.
Applications include homology-directed repair (HDR) and episomal expression.
The flexible nature of Touchlight’s novel circular DNA molecules allows for bespoke designs to optimise gene therapy platforms that utilise DNA payloads for episomal expression or integration.
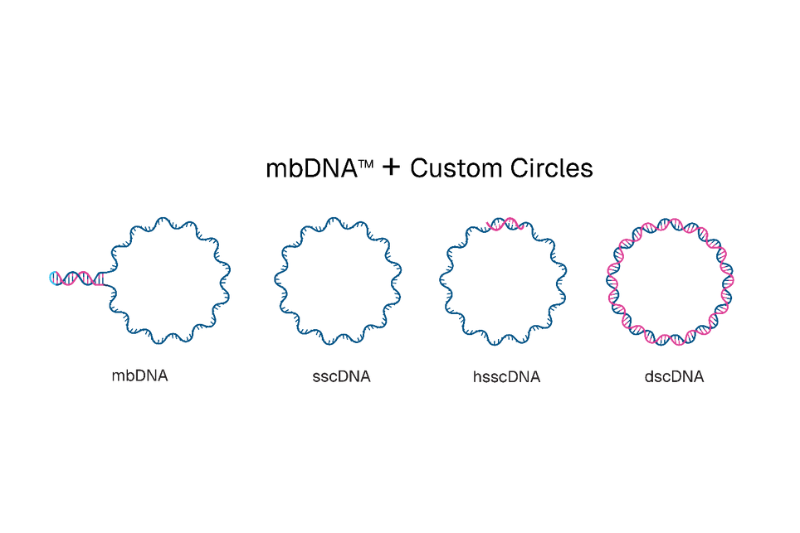 sscDNA is a fully single-stranded circular DNA molecule, whereas hsscDNA contains bespoke double-stranded region(s) of user-defined length.
sscDNA is a fully single-stranded circular DNA molecule, whereas hsscDNA contains bespoke double-stranded region(s) of user-defined length.
dscDNA is a fully double-stranded circular molecule.
The sequences of all DNA constructs are completely user-defined and ideal for transposases, recombinases, HITI and episomal expression.
“mbDNA is a market-leading technology providing the best-in-class HDR template available today,” said Karen Fallen, CEO at Touchlight.
“With the addition of sscDNA, hsscDNA and dscDNA, we’re empowering researchers and developers to unlock new possibilities in gene editing and therapeutic innovation.”
