Asahi Kasei announced that it will launch two new speciality grades in its Sonanos portfolio.
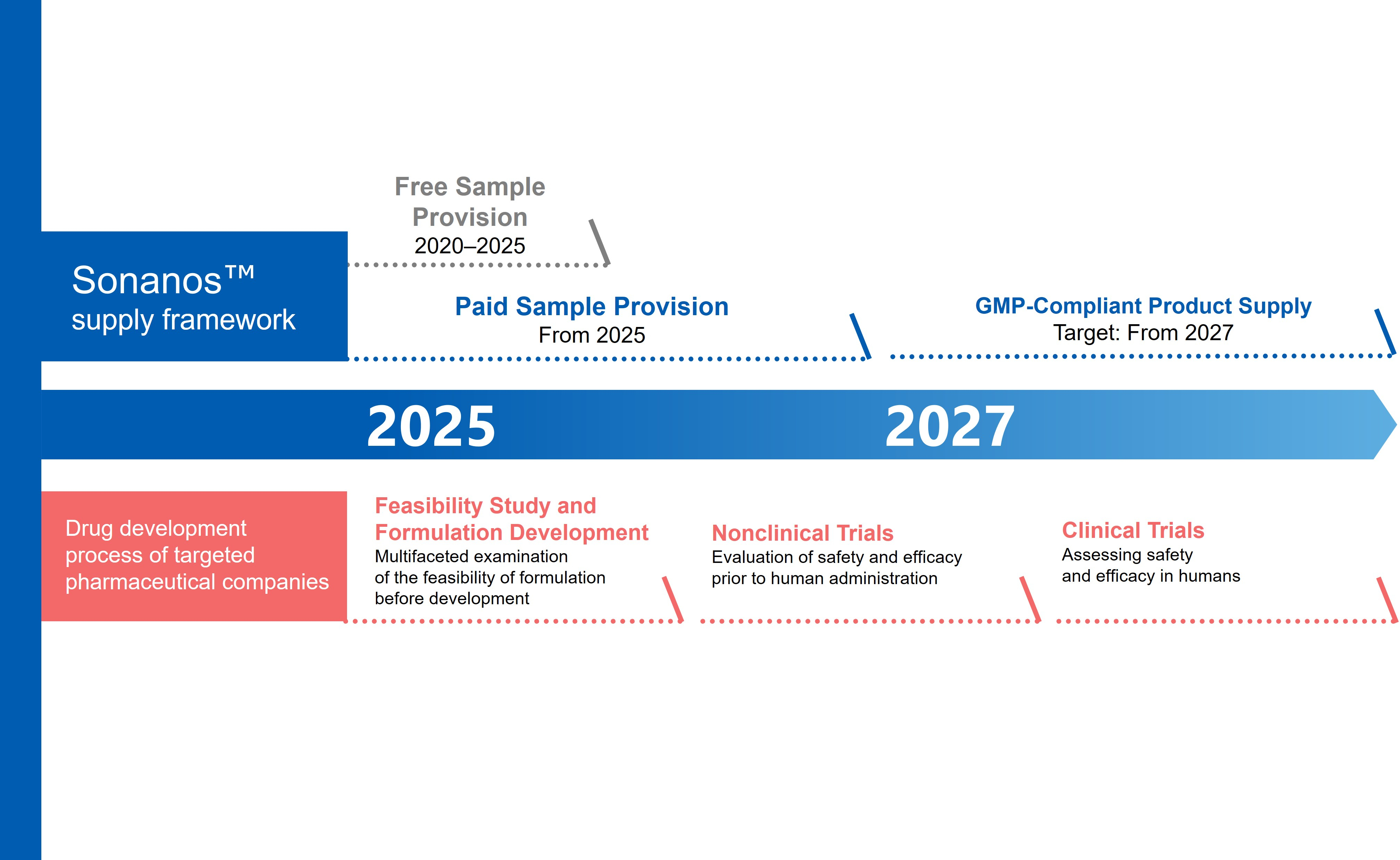
Paid samples are already available and products manufactured in compliance with Good Manufacturing Practice (GMP) are scheduled for sale in 2027.
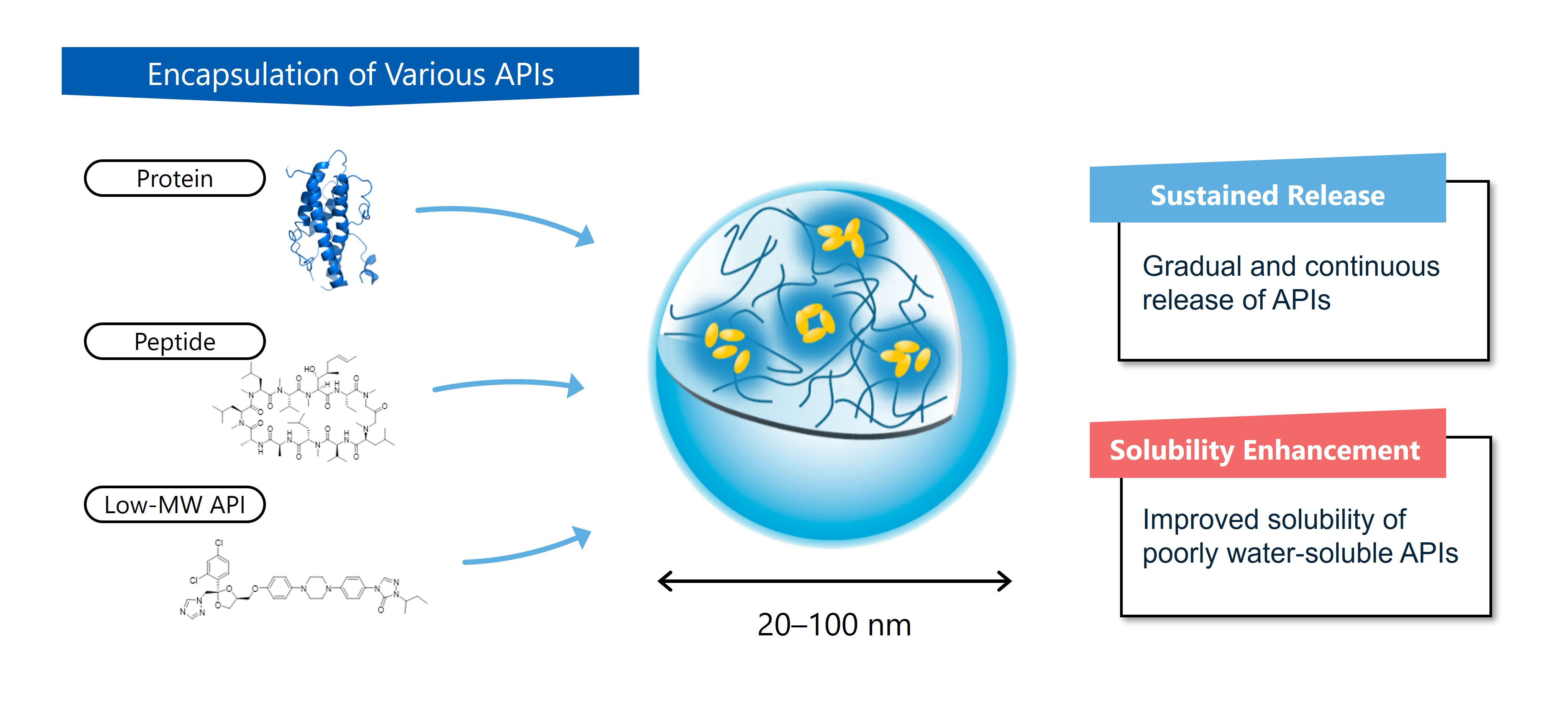 Sonanos is a next-generation excipient designed to improve the formulation of injectable drugs.
Sonanos is a next-generation excipient designed to improve the formulation of injectable drugs.
This aligns with the company’s long-term growth strategy to enhance its presence in the fast-growing pharmaceutical materials market.
Global demand for biologics, peptides and oncology therapies is rapidly increasing, creating pressure on pharmaceutical companies to solve formulation, drug persistence and delivery challenges.
Sonanos addresses these needs by enabling sustained release and enhanced solubility.
These are key capabilities that can achieve long-acting injectables, formulate poorly water-soluble compounds and expand therapeutic possibilities through overcoming formulation challenges.
Since 2020, Asahi Kasei has partnered with global pharmaceutical companies to conduct more than 60 feasibility studies using Sonanos samples.
Based on these collaborations and understanding of rising industry challenges, the company has introduced two new speciality grades, including the following:
- Sonanos PG: optimised for sustained release of biologics and peptides while supporting patient-friendly dosing
- Sonanos DS: designed to enhance the solubility of poorly water-soluble active pharmaceutical ingredients (APIs).
 Each of the new grades was developed through extensive optimisation, including the ability to encapsulate higher concentrations of active ingredients, based on experience with earlier prototype samples.
Each of the new grades was developed through extensive optimisation, including the ability to encapsulate higher concentrations of active ingredients, based on experience with earlier prototype samples.
With samples of both grades with guaranteed analytical values now available for non-clinical development, Asahi Kasei is laying the foundation for broader adoption.
The company plans to supply products manufactured in compliance with GMP in 2027, meeting international guidelines for pharmaceutical excipients and impurities, which is necessary for clinical development.
“Sonanos represents a significant step forward for our organisation and healthcare focus,” said Hideyuki Kimura, Senior General Manager of Asahi Kasei’s Healthcare Materials Division.
“This expanded lineup of our excipient products deepens Asahi Kasei’s role in the pharmaceutical industry through critical excipient offerings that address customer needs in novel ways.”
Sonanos is also advancing through Asahi Kasei’s spin-out venture, DiveRadGel, which applies the technology to cancer vaccine development.
The vaccine-grade Sonanos DV has already entered GMP production to support early-stage clinical trials, demonstrating the platform’s commercial and therapeutic relevance.
Asahi Kasei’s Healthcare Materials Division also offers microcrystalline cellulose (MCC) excipients such as Ceolus and Celphere.
Sonanos, along with these MCC products, will be featured at AAPS 2025 PharmSci 360, held in San Antonio, Texas, from November 10-12, at booth #3443.
