Ahead of CPHI Milan – the world’s largest pharma event, held at Fiera Milano [8-10 October] – new insights from the CPHI Annual Industry Survey* reveal that the pharma sector is on the cusp of a major shift, with approvals for complex molecules expected to outpace those for small molecules for the first time. In 2023, of the 55 new drug approvals, nine were for tides (five peptides and four oligonucleotides), with a further 17 biologics.
This shift is already influencing decisions within the CDMO sector, with biologic and Tides-focused CDMOs projected to be the most lucrative manufacturing over the next five years. Nearly 300 companies were surveyed, each selecting up to two modalities. Biologics (53%) and Tides (43%) emerged as the top choices for CDMO investment, viewed as the most promising areas to secure the best returns over the next five years. Last year’s modality de jour, ADCs surprisingly finished last achieving just 15% of the vote, while small molecules remained consistent with 35%*.
Tara Dougal, Brand & Content Director at CPHI Milan, commented: “We are observing this trend first hand at CPHI Milan, both in terms of exhibitor evolution and in the demand for increased biological and complex therapy focussed content. Notably, there has been no contraction in the small molecule CDMO space. Instead, we've seen a steady influx of new CDMOs at CPHI, with many mid- and large-sized CROs and CDMOs also investing in capacity for both Tides and mAbs.”
CPHI Milan will welcome more than 62,000 pharma professionals, 2500+ exhibitors and more than 600 CDMOs – making it by far the largest gathering in the contract services annual calendar.
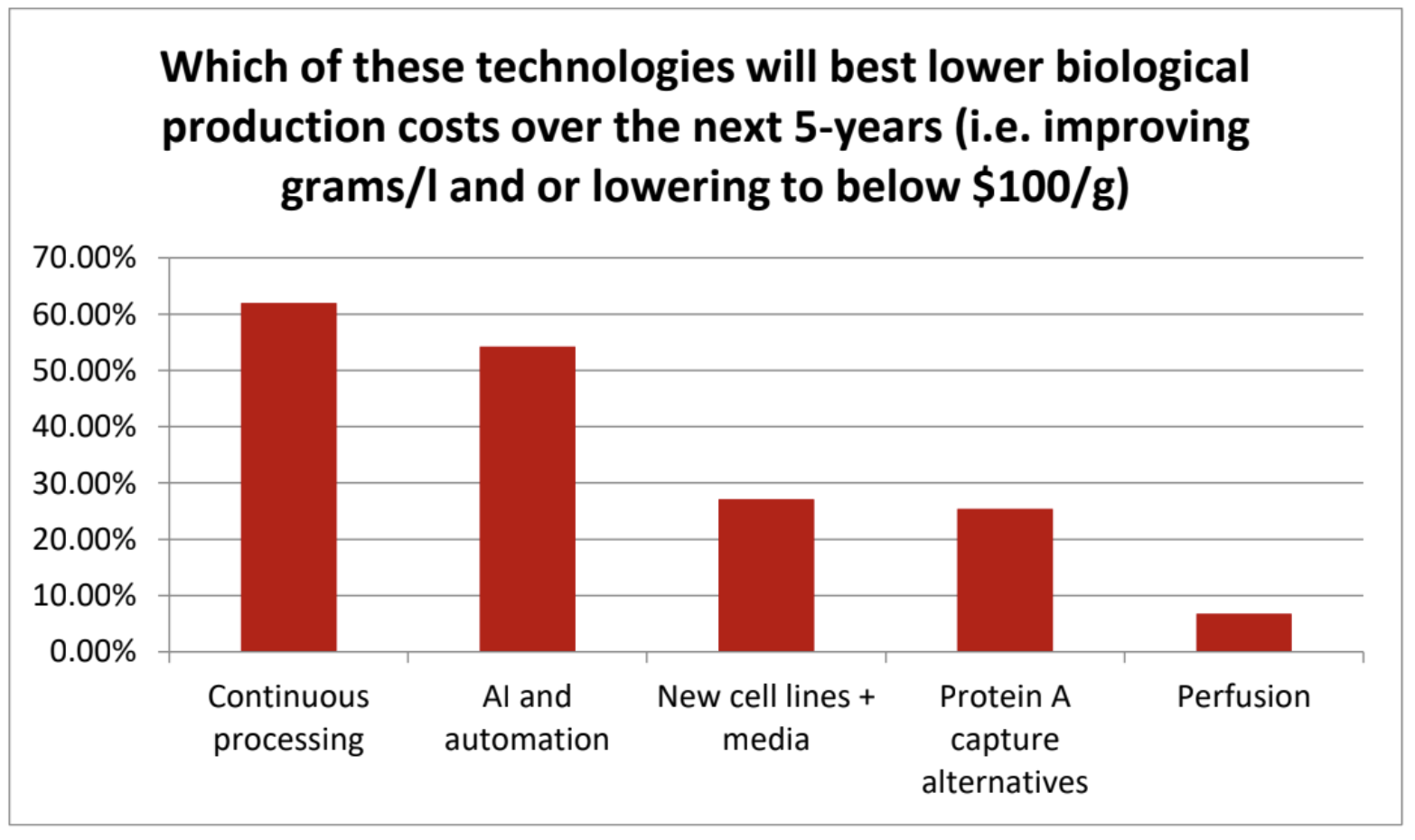
One particularly significant finding is that efforts to reduce the cost of monoclonal antibody (mAb) manufacturing are accelerating, with 62% identifying ‘continuous bioprocessing’ as the leading technology to lower production costs over the next five years. This far surpasses perfusion (7%) and new cell lines (27%), which were the primary methods of improving yields (grams/L) and reducing costs in the previous five years**.
Dougal added: “We’re seeing that alongside changes in the drug discovery pipeline, our bio exhibitor base at CPHI Milan is growing. With newer modalities requiring sophisticated manufacturing approaches and a high-level of technical expertise, choosing the right outsourcing partner is extremely important for the road to commercialisation.
It’s why we have seen many new audiences at the event as these drugs are often developed by biotechs and/or in smaller collaborations that need wider contract services networks to advance. CPHI Milan is now an integral component of the industry’s development pathway, and we are consistently looking at new ways for both our attendees and exhibitors to meet more potential partners. We see attendees sourcing new partners much earlier than before to meet capacity requirements, while also using the event to secure alternative supply options, as well as secondary and even tertiary partners.”
CPHI Milan 2024 will also celebrate its 35th anniversary, offering unparalleled networking and access to the global supply chain, with representatives from nearly 170 countries. The event will feature a dedicated Bioproduction zone, over 150 speakers and more than 100 content sessions. Highlights include the Next-Gen Bio Theatre, which will host sessions on mRNA vaccines for advancing immunity, a roundtable on global bio-manufacturing trends, and a keynote exploring the EU's biotech and bio-manufacturing strategy.
*280 company respondents taken in August and September
*Table 1
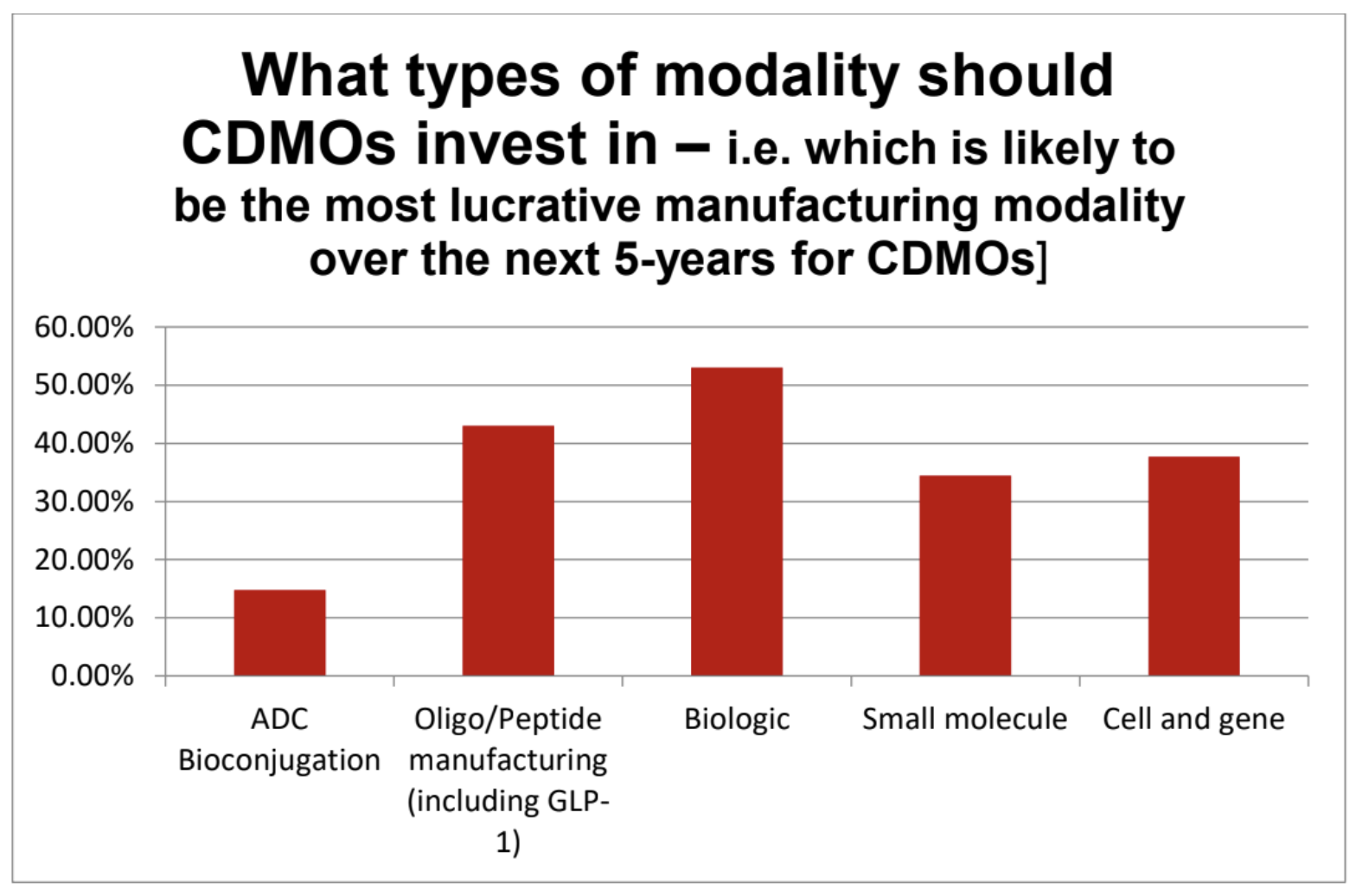
**Table 2