Bio-Equivalence (BE) studies are a necessary part of Licensing pharmaceutical products in many parts of the world. Whilst the exact requirements may differ, the objective of BE studies is clear and simple: to prove that the product to be licensed is equivalent to the reference product.
However, arranging, executing, analysing and submitting the results of a BE study can be extremely complex and time consuming, as well as costly.
Rephine’s experience within Clinical Trials enables it to provide a total service that can ensure an efficient, speedy and cost effective solution to BE studies.
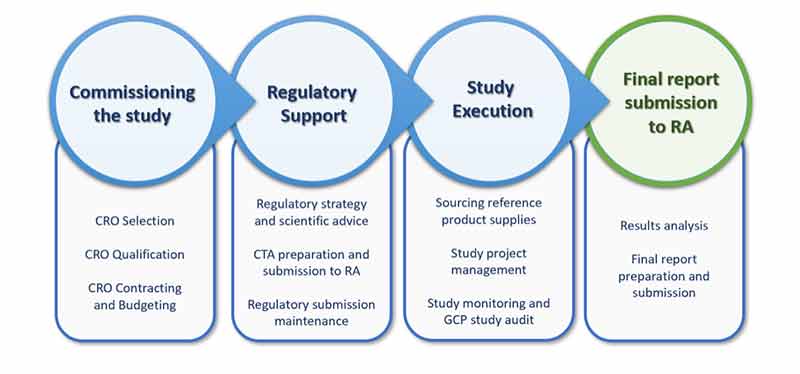
Rephine’s services for a BE study begin with CRO selection and qualification at commissioning of the study. During execution of the study Rephine’s top-tier experts can support sponsors with a comprehensive range of services, including project management, study monitoring and GCP audits. In addition, through the entire program, Rephine’s highly experienced consultants can provide a full range of regulatory activities up to the final report submission to Regulatory Authorities.
Contact Rephine at enquiries@rephine.com or call 0044-1763-853135 to discuss your requirements and learn more about how Rephine can help you design and execute a world-class Bio-Equivalence study.
