Extracting intracellular products such as DNA, ribosomes and mitochondria allow further study and understanding of how cellular mechanisms work and the ways they respond to different environments. The cell membrane acts as the barrier between the user and desired product, with an additional layer in the form of a cell wall in the case of plant cells, fungi, bacteria, algae and archaea. It is therefore required that cells are first lysed to extract the intracellular products.
High pressure homogenisation is an efficient means of cellular disruption, whereby a sample is subjected to ultra-high pressures as it is forced through a narrow orifice. As the sample exits the orifice, stress forces such as cavitation and shear are generated; lysing the cell.
Avestin’s range of EmulsiFlex high pressure homogenisers can be specified to function at pressures towards 45,000psi, and thus lyse the toughest of samples. With fully adjustable pressures, and access to components of stringent temperature control, Avestin’s first rate portfolio can be tailored to specific operator criteria i.e. identification of the optimal processing parameters, in order to achieve the highest possible yield.
Intracellular contents are typically temperature-sensitive, and so it is crucial to control and limit the product exposure to temperature increase. Whether at R&D scale or production scale, Avestin offers efficient mechanisms of sample cooling.
For less stringent, but more commercially viable control, Avestin offers heat-exchanging cooling coils, which use their large surface areas to extract heat quickly, and nullify the temperature increase. Where requirements focus on more accurate control (<10°C), Avestin offers sanitary heat exchangers, which act as an efficient cooling jacket.
The sheer speed of the high pressure homogenisation technique offers a distinct advantage over other lysis methods
In the case of protein extraction, temperature control may be critical to minimising the effects of proteases. Proteases are released during cell lysis, which may digest some types of intracellular proteins. Keeping the sample cooled slows down this digestion, and in addition, appropriate protease inhibitors may be added to the solution. The sheer speed of the high pressure homogenisation technique offers a distinct advantage over other lysis methods in that the time target proteins are broken down by proteases is very short. In other words, it allows a high yield in a short time.
Another method of cell lysis is enzymatic lysis, often via lysosymes. This is sometimes applied to bacteria, as lyosymes hydrolyse the glycosidic bonds in peptidoglycans found in the cell wall1. However, this method is not very effective against gram-negative bacteria, due to the cell wall being surrounded by an additional outer lipopolysaccharide membrane. Upon cell lysis, nucleic acid material is released, which increases sample viscosity, and so DNase is often required to reduce this problem. On a large scale, the lysosyme and DNase can become expensive, and so the methods tend to become less commercially viable for the user.
Sonication, like high pressure homogenisation, uses mechanical forces to disrupt bacterial cells. Direct sonication involves a metal probe which produces ultrasound to induce localised low pressure regions that induce shear stresses and thus cell lysis. During sonication, excessive heat can be generated, and so the ultrasonic pulses are given in short bursts. This can be time consuming, and in addition, for cell quantities larger than 50g, the method is not as effective as high pressure homogenisation, due to the difficulty in maintaining low temperatures, and the long sonication times to achieve a high percentage of lysis2. This extended processing duration poses a risk to the samples, as the high-energy created via sonication can cause protein denaturation via heat. With larger samples, there is an increased chance that the probe will not affect all cells, and so the process efficiency would decrease.
A recent study compared and evaluated the performance of various cell disruption methods for the release of recombinant hepatitis B core antigen (HBcAg) from E.coli3. As shown in Figure 1, (below), high pressure homogenisation was able to produce the greatest extent of disruption, and the highest yield of HBcAg.
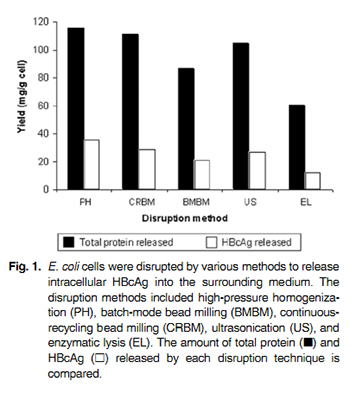
Since high pressure homogensation can efficiently lyse cells without the aid of solvents and enzymes, the extracted proteins are more likely to be in their natural form compared to other methods. In other words, they are far less likely to alter in shape. High pressure homogenisation is widely the preferred method for cellular disruption, due to greater yield of viable product.
In addition, high pressure homogenisation has additional capabilities in particle size reduction, nanosuspensions and liposome extrusion. Avestin offer high pressure homogenisers from bench-top scale to production-scale systems, capable of processing up to 1,000L/hr. The systems are suitable for GMP, and have near-perfect scalability; optimising reproducibility.
For more information on the Avestin range, visit www.biopharma.co.uk or contact Ashley Morgan on amorgan@biopharma.co.uk | +44 (0)1962 841092
References:
1. Peternel, S. and Komel, R. (2010). Isolation of biologically active nanomaterial (inclusion bodies) from bacterial cells. Microbial Cell Factories. 9:66.
2. European Molecular Biology Laboratory. (.). Protein Purification: Extraction and Clarification. Available: https://www.embl.de/pepcore/pepcore_services/protein_purification/extraction_clarification/cell_lysates_ecoli/. Last accessed 19th Sept 2016.
3. HO, C.W., Tan, W.S., Yap, W.B., Ling, T.C. and Tey, B.T. (2008). Comparative evaluation of different cell disruption methods for the release of recombinant hepatitis B core antigen from Escherichia coli. Biotechnology and Bioprocess Engineering. 13 (5), 577-583.
*Figure 1 was acquired from reference number 3.

