During the early planning phases, it’s important to conduct a thorough review of the chemical API synthesis plant’s production profile and technological process functions to be able to design an efficient and effective facility.
API production using chemical synthesis processes seemed to go out of fashion in the European pharmaceutical industry during the last decade, with a number of construction projects tending to focus on biotechnological production.
Now, however, in recent years, several new projects for the development of API synthesis plants have emerged in Europe, particularly for the production of highly potent APIs (HPAPIs). In the coming years, the market for HPAPIs is expected to expand at a CAGR of 8.3%.1,2
Current API production plant projects have shorter implementation times and faster times to market, which goes hand in hand with the trend for more individualised API products and smaller production volumes. As such, the integral engineering procedures for these chemical synthesis facilities need to be further developed to meet these requirements.
Some basic considerations when designing a chemical API synthesis plant include the following:
- a very real trend in pharmaceutical production is continuous manufacturing; however, in chemical API synthesis, batch-based processing still dominates because it’s easier to control the thermodynamic and kinetic parameters of the chemical reactions3,4
- API/HPAPI annual production volumes are often quite low
- special materials are frequently required for reactors, pipes and seals, etc.
- reactants and catalysts often have different aggregate states (solid, liquid, gaseous)
- scale-up is sometimes difficult
- chemical API syntheses often use several successive steps with intermediate purification processes
- the GMP requirements can vary for different synthesis steps
- containment measures have to be planned individually for each step and each compound
- safety requirements can vary for different syntheses (special caution is needed when dealing with exothermic reactions)
- explosion protection measures must be included in the overall plant design
- cleanroom and HVAC requirements must comply with specific GMP guidelines and may vary for different synthesis steps
- water-based cleaning may be ineffective; the resulting contamination or cross-contamination of APIs could result in unexpected side reactions.
Figure 1 shows a typical example of the technological processes involved in one synthesis step. In this example, API separation was done using a basket centrifuge and the drying process involved a double conus dryer.
Equipment occupancy estimates were done for all the process operations, including machine filling and emptying, heating and drying, and it transpired that the crystallisation vessel was the production line bottleneck.
As a result, the insertion of a second crystallisation vessel both increased the overall efficiency of the entire process and the yearly capacity of the plant. The utilities concept, layout, cleanroom and HVAC design were developed for the whole API production plant. Expansion options for future capacity growth should be integrated at an early stage and incorporate all of the above planning disciplines, as well as civil design.
Production in dedicated synthesis trains
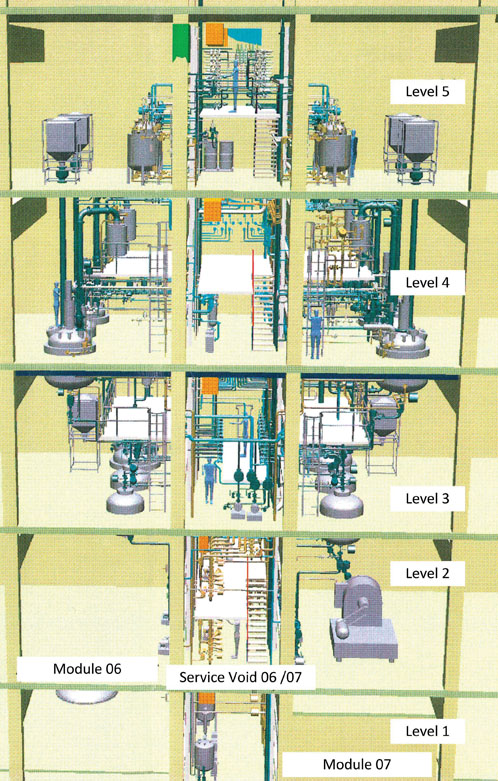
Figure 2: 3D model of an API production plant with separate synthesis steps
The separation of each step in an API synthesis production plant into dedicated, separate trains is a well-known approach (Figure 2).
The concept of parallel synthesis trains is often preferred in single product plants. As the separated process trains of the API plant work independently, cross-contamination must be strictly prevented.
The successive synthesis steps can only be started if the quality parameters of the intermediates are confirmed by the required QC analysis. Having said that, it is actually possible to produce different APIs in parallel production trains. The advantages of separate synthesis trains are as follows:
- the type and size of dedicated equipment can be optimised to specific reaction volumes, material properties and individual process times
- equipment occupancy and debottlenecking can be done individually for each synthesis step
- containment measures only have to be considered when really necessary
- cleanroom and HVAC design are individually designed for each synthesis train as required by GMP
- health and safety measures can be implemented more specifically and stringently.5
As the supply functions — as well as product and by-product handling — can be shared, these units can mostly be handled as dedicated modules (Figure 3).
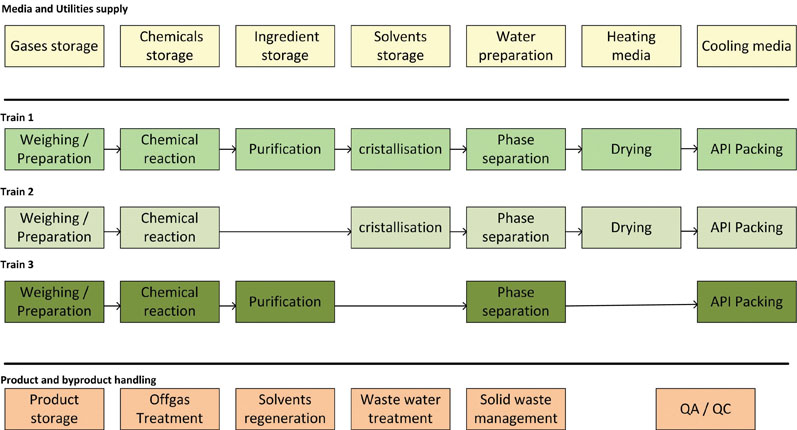
Figure 3: Typical chemical API synthesis functions with parallel synthesis trains
For instance, the function of waste water treatment is often done using preassembled modules.6 Engineering costs and time can be significantly reduced by using a modular approach.
Of note, though, both investment and operational costs for this type of API synthesis plant can be considerable. At the same time, the flexibility of this type of plant is very low, so such designs are favoured for single-product facilities that require high yearly API capacities.
Modularisation of API synthesis
The production programmes of specific plants can vary quite widely and, as a result, multiproduct plants have become more popular, as has the modularisation of pharma production facilities.7–9 Benefits such as lower investment costs, shorter planning times and higher production flexibilities can also be achieved by modularising the core synthesis process too.
In Figure 4, one example for a modular approach is shown. The plant consists of three reaction modules of different sizes and types. All of the other process modules can be connected by hoses or pipe junction boxes, if required, and a variety of purification operation types (such as filtration or extraction) can also be integrated.
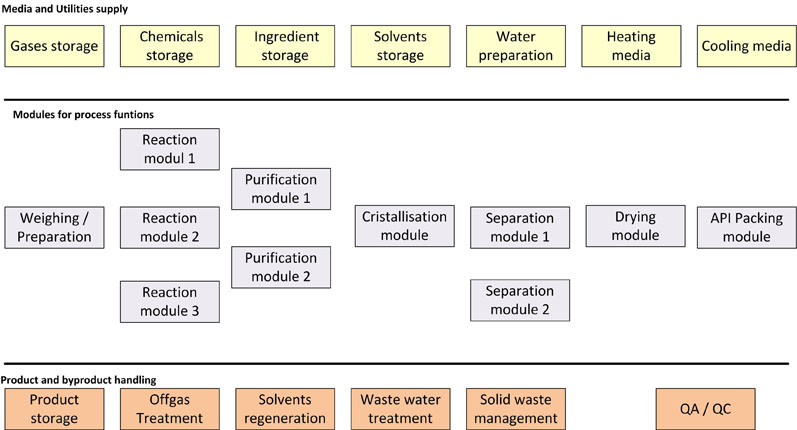
Figure 4: The modular design of chemical synthesis processes
The separation modules in this example could include filtration or centrifugation, for instance.
The modular design enables a high level of flexibility to be incorporated into the production configuration. As such, this approach is often used in contract manufacturing plants for small-scale production, in multiproduct plants or for the production of clinical batches.10,11
The modular approach facilitates production capacity scale-up and makes maintenance easier. Process optimisation and updating the existing production regime is enabled by the judicious selection of appropriate module connections.
Careful analysis of the synthesis process is crucial during the early phases of a project to accurately define the equipment sizes and batch times for a modular plant. Reference processes are often specified by the manufacturer as the basis of the design.
Otherwise, standard configurations can be used to design modules for processes that are under development. If additional syntheses are destined to be implemented in an existing plant, all the requirements for containment, safety, GMP and cleanroom compliance will need to be explicitly approved for the new process(es).
There are, however, some disadvantages associated with the use of a modular plant. The type, size and occupancy of the process equipment in a modular plant are not specifically optimised for a dedicated API production step. Strict cleaning procedures are required to minimise the risk of cross-contamination in modular API plants and well-defined standard operating procedures (SOPs) must be in place to minimise the risk of operator error.
Automation concept for modular plant design
Any potential errors and confusion can be avoided by using electronic recipes for certain operations and an appropriate batch recording system.
An intelligent and flexible automation approach is crucial to combine the telemetry from the various modules of the plant into one database and remain compliant with current GMP requirements.
For validation purposes, any robust automation system should integrate the output from the individual process modules and package units, as well as including computerised systems.
The ISPE’s guide, GAMP 5: A Risk-Based Approach to Compliant GxP Computerized Systems, published in February 2008, provides pragmatic and practical industry guidance regarding how to achieve compliant computerised systems that are efficient, effective and fit for purpose (and meet the requirements of 21 CFR Part 11 for batch recording and electronic signature).
With the development of autonomic, decentralised systems, which are able to monitor the activities of modules from afar (see Namur NE 148), several new ways to increase the flexibility of these facilities and shorten project durations are becoming popular, as well as providing a way to support the automation of a modular plant throughout its entire lifecycle.12–14
Extant models focus on uniform, open and manufacturer-independent communication systems and interface architectures for the integration of processes in existing automation and packaging units. The intention is to enable the flexible integration of intelligent modules with their own, onboard, automation systems into complete lines.
The development of recognised automation standards is a basic requirement for modular pharmaceutical production. Interfaces with package units, modules and separate measuring points have to be standardised.
In fact, the descriptions of single process functions — assigned by engineers — are very important for the overall automation concept. The functional descriptions of the reaction/crystallisation modules, for example, comprise some of the most critical parts of automated API synthesis.
References
- www.transparencymarketresearch.com/high-potency-active-pharmaceutical-ingredients-market.html.
- https://bit.ly/2KmjUBW.
- www.manufacturingchemist.com/news/article_page/Continuous_processing_the_future_of_pharmaceutical_manufacturing/142250.
- www.manufacturingchemist.com/news/article_page/Continuously_driving_pharmaceutical_manufacturing_efficiencies/148386.
- www.cleanroomtechnology.com/news/article_page/Highly_potent_APIs_hazards_in_classification_and_handling/141694.
- www.process.vogel.de/auf-dem-pruefstand-flexible-produktion-und-modularisierung-v-39111-2791.
- www.process.vogel.de/vom-kern-zur-huelle-wie-modulare-planung-flexibilitaet-in-die-pharmaproduktion-bringt-a-722197.
- D. Steinhäuser, Glatt GIT, “Modular Plant Concepts for Biotechnological and Pharmaceutical Production,” presentation at ACHEMA Congress 2018 (13 June 2018, Frankfurt, Germany).
- www.process.vogel.de/so-viel-potenzial-bietet-der-modulare-anlagenbau-a-716925.
- www.chemanager-online.com/en/topics/management/modular-every-respect.
- www.chemanager-online.com/themen/management/modularitaet-bringt-flexibilitaet.
- www.process.vogel.de/dezentrale-intelligenz-fuer-modulare-anlagen-a-708342.
- www.process.vogel.de/die-dima-methodik-dezentrale-intelligenz-fuer-modulare-anlagen-entwaechst-ihren-kinderschuhen-a-554253.
- www.process.vogel.de/modulare-automatisierung-fuer-mehr-flexibilitaet-a-713317.
N.B. This article is featured in the December 2018 issue of Manufacturing Chemist.
