From its earliest applications in the stabilisation of blood plasma, freeze drying has been in use in the life science industries for decades. During this period, the freeze dryer — or lyophiliser — has evolved from a simple device for vacuum drying at low temperature to an extremely sophisticated integrated system. Current options combine several processes to ensure that a product is consistently delivered to technical and biological specifications while also considering economic, safety and environmental issues. In the dynamic landscape of pharmaceutical manufacturing, automating this process has played a pivotal role in terms of ensuring compliance with stringent health and supervisory regulations.
ALUS — the automatic loading and unloading system for lyophilisers — is designed to provide maximum flexibility in terms of containment, layout and product flow, incorporating loading and unloading from either one side or two (pass through design). ALUS’s primary objective has been to automate production processes, reduce human intervention in sterile environments and mitigate contamination risks.
The journey began in 1989 with the introduction of the inaugural Transfer Cart. Throughout the 1990s, GEA developed the first fixed push-pull system and, with time, the escalating requirements of health authorities for aseptic manufacturing and contamination prevention fuelled the further evolution of ALUS. Strategic collaborations led to the integration of barrier technologies, culminating in the development of the world’s first ALUS under an isolator in 1997. This breakthrough allowed for full adaptability in terms of product flow and building design.
Throughout the 2000s, design optimisation, a reduced footprint, the seamless integration of loading/unloading, the elimination of moving parts above the product and enhanced cleanability have all been prioritised through patented designs. Keeping building costs to a minimum and making integration with retrofit applications much easier were key drivers.
In 2014, the European Union (EU) ATEX directive outlined requirements for manufacturers to ensure that their equipment meets safety and quality standards for use in potentially explosive atmospheres. GEA’s ALUS fully aligns with these standards.
Total vial traceability: LYODATA
In collaboration with SCHOTT and HEUFT in 2016, GEA developed a vial traceability solution to address the EU’s drug anticounterfeiting directive. This solution safeguards the rights of trademark and patent holders and ensures patient safety by preventing counterfeit drugs from entering the market.
On-the-go online moisture control: LYOSENSE
In 2018, the focus shifted to real-time product control with the development of LYOSENSE in collaboration with CA INDATECH. This online measuring device, based on multipoint NIR measurements, offers a comprehensive and non-destructive evaluation of freeze-dried product cakes in real-time. This easy-to-install and use online measuring device is a fast and non-invasive solution to moisture detection/control that both ensures cake homogeneity and assesses critical process parameters.
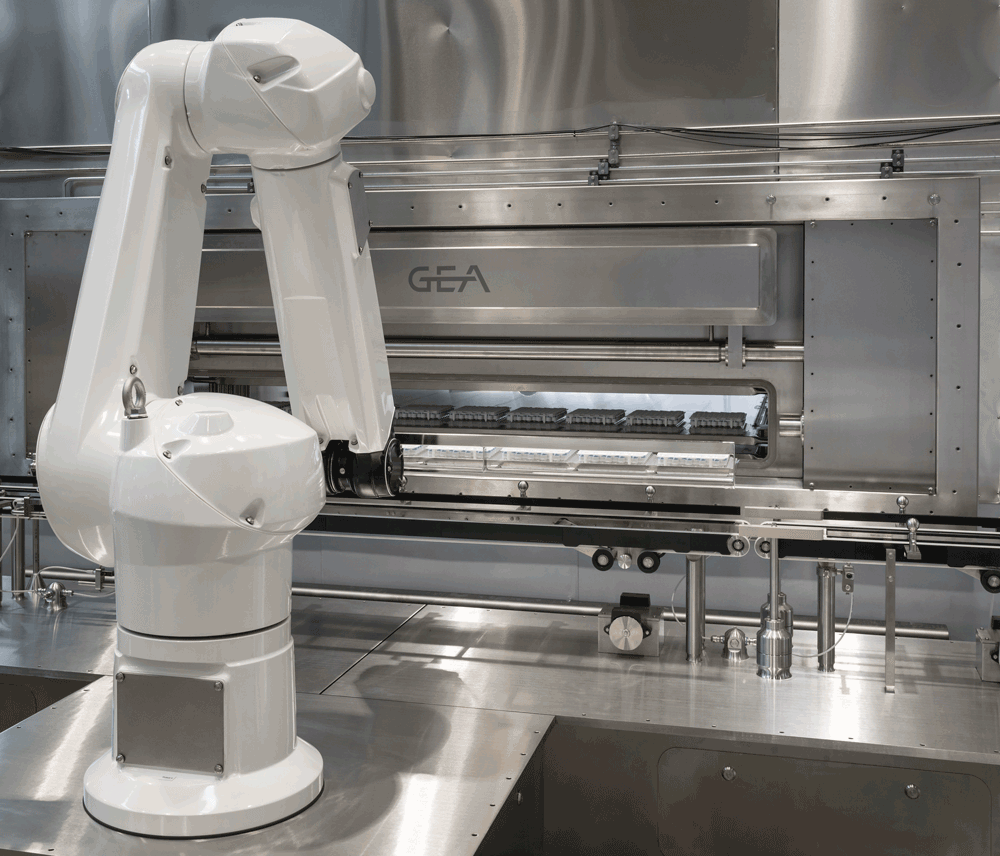
ALUS Robot
As the pharmaceutical industry gears up to implement the European Commission’s revised Annex 1 good manufacturing practice (GMP) guidelines (as per August 2023), GEA has already taken a significant step forward. The introduction of the ALUS Robot aligns with the industry’s commitment to increasing product protection and eliminating direct human interventions. Robotic automation ensures error-free product handling by utilising nests to prevent container fall, minimise glass-on-glass contact and increase processing speeds to up to 1000 vials per minute in larger systems. In response to the new Annex 1, which becomes effective for lyophilization-related applications from August 2024 onwards, GEA is ensuring future compliance by providing relevant Contamination Control Strategy (CCS) documents, including CFD studies, GMP risk analysis and rationale-based monitoring concepts.
Future-ready technology
Now, more than ever, hygienic design has become a crucial element of pharmaceutical production. The newly introduced requirements increasingly emphasise the avoidance of plant and procedures being positioned or taking place above critical operations to prevent the possible contamination of sterile products.
With the ever-rising expense of researching and developing pharmaceutical dosage forms, even the smallest amount of product loss has become very undesirable. Significant progress has been made by using nests to handle vials and syringes, which avoids breakage, cosmetic defects and particle generation.
In addition, cleaning processes — whether manual or automatic — should be validated, and the process and system design must permit cleaning validation. Isolators are the preferred technology when it comes to handling sterile products, with RABS now requiring additional justification to authorities. The flexible Transfer Cart system with RABS containment substantiates its use in situations when pharmaceutical production demands maximum flexibility.
In summary
Since 1989, GEA has been at the forefront of ALUS technology. Having designed, patented, manufactured and made continuous improvements to the ALUS system, it’s the first company to develop this technology, create fixed systems (conveyor-pusher systems) and subsequently integrate them into an isolator. The company’s 35-plus years of experience have resulted in a validated base of more than 350 production-scale ALUS systems being installed worldwide, seven patents and an ongoing programme of improvements to enhance both handling and maintenance. But that’s far from the end of the story. There’s more to come and next-generation ALUS products are already being developed. Watch this space.

