Capping is a term used to describe the detachment of a cross-sectional fragment from the face of a tablet. This usually occurs just after the tablet has been ejected from the compression die and will invariably lead to rejection.
For a registered product, changing the formulation is not the preferred option to resolve such issues. Moreover, for a high-dose product, there are limits to formulation changes and the final solution may not fully resolve the capping problem.
Yet, an alternative solution may now be available from GEA.
Why does capping occur?
If a given substance is exposed to a pressure load, it will react in several different ways. If a modelling material is deformed by mechanical energy, for example, the mass will maintain its form even when the external force is no longer applied; this is called plastic deformability.
If a spring is deformed mechanically, it will return to its initial state even when the external force is no longer applied; this is referred to as elastic deformability.
If a mechanical load is applied to, say, cornflakes, the phenomenon of brittle fracture occurs. And, finally, there’s the issues of viscoelasticity, which is a combination of the previously mentioned reactions. This describes substances that react either plastically or elastically in a time-dependent manner.
The extent to which a tablet is prone to capping depends on the deformation behaviour of individual components.
If materials are used that deform plastically or undergo brittle fracture, the risk is low. But, if the tablet formulation contains substances that deform elastically or demonstrate viscoelastic deformation, there is a high risk of capping, particularly with fast-running production-scale tablet presses.
The situation is exacerbated if the active pharmaceutical ingredient (API) itself shows this behaviour and must be incorporated into the tablet in high concentrations.
In almost all other cases, capping can be avoided completely by the appropriate choice of pharmaceutical excipients. However, capping will always occur if, following compression, more elastic energy is accumulated in the tablet than its inner structure can absorb.
Apart from the choice of excipients, the processes that precede tableting also influence the tablet’s tendency to cap. In the case of direct compression, only the compression properties of the substances used will define the extent of the capping potential.
Another problem associated with direct compression is the higher proportion of fines, which also increase the tendency to cap. Wet granulation, by contrast, enables capping to be minimised, according to how evenly the binder is distributed during granulation.
Therefore, granulates that have been produced by spray granulation generally cap less than those produced using an intensive mixer granulator.
Another cause of capping is entrapped air that has been compressed during main compression and eventually shatters the tablet because of perfect elastic behaviour.
The more open-pored a material is — usually discernible by its low bulk density — the more air it contains. Most of this air should be removed during precompression. Yet, the problem here is that with faster tableting speeds, less time is available.
Overcoming entrapped air issues has long been addressed, however, with GEA’s Air Compensator. Fitted to the pre-compression station of every GEA press — including the NexGen Press range — this unique device comprises a piston-mounted roller that can move within an air cylinder.
This allows operators to modify the pre-compression force. When combined with an extended dwell time, prior to the main compression event, the mechanical strength of the resultant tablets is improved, which reduces the risk of capping.
Of cores and coating: the influence of tablet shape
In what he calls the “good old days,” explains Dr Harald Stahl, Senior Director for Innovation and Strategy, “we used to make tablets that were coin shaped or flat faced — perfectly cylindrical with slightly polished edges. However, for cosmetic reasons and to make them easier to swallow, coated tablets became the norm.”
“But you can’t coat coin-shaped cores with conventional tablet coaters because the edges chip off and they tend to stick to each other; that’s why we — as an industry — developed the now commonplace dome shaped or biconvex form. Unfortunately, that topography is one of the root causes of capping.”
To progress from a powder to a granule to a tablet, Harald notes, you need some binding forces. There are three that play key roles in compression. First, there is the binder itself, which is introduced during wet granulation (if applicable).
Then, there are hydrogen bonds, which are affected by humidity (more humid equals better binding) and there are Van der Waals forces, which heavily depend on inter-particulate distance: the closer the individual particles are together, the stronger the bonds become.
When you compress a coin-shaped tablet, you go from a density of, for instance of 0.5/0.6 to 1.0 g/cm3. What’s important, however, is that every granule is compressed to the exact same value throughout the entire cross-section.
By contrast, with a dome-shaped tablet, the particles at the radial edges are compressed more than those within the actual dome (Figure 1).1 As such, the Van der Waals forces are much weaker in this section of the pill and why capping occurs.
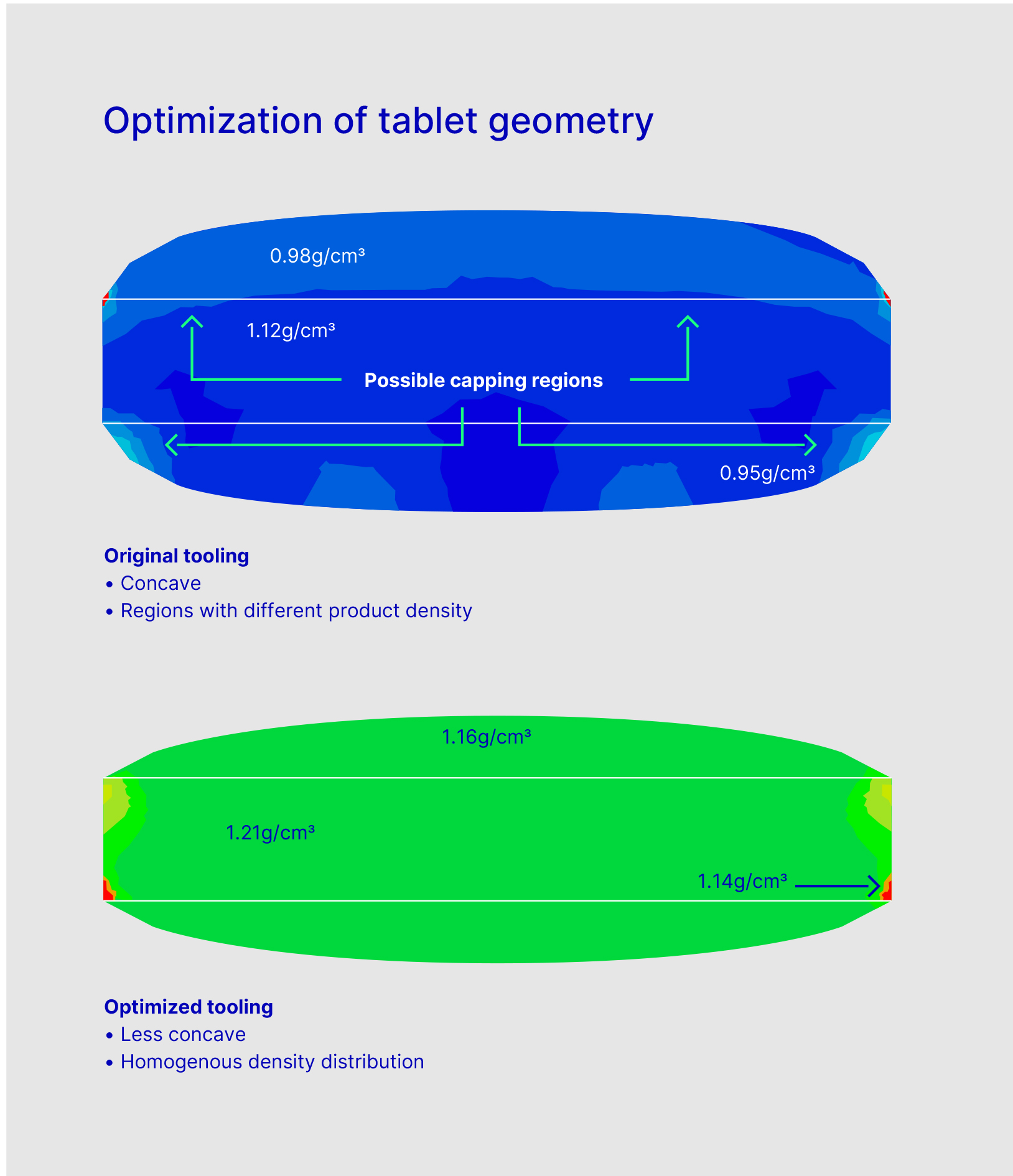
Figure 1: Biconvex tablets with different regions of particle density (top = standard tooling, bottom = optimised tooling).
A downstream solution
With that in mind, and what might not currently be well known, is that it is now actually possible to coat coin-shaped tablets. The GEA Coater is an all-in-one solution that’s capable of research, clinical and full-scale production using the same system.
It can be included in both batch and continuous production lines, either as a standalone unit, in bin-2-bin systems or linked to any up- or downstream process
Designed to gently and accurately deposit controlled amounts of coating materials onto tablet cores — even if they are hygroscopic or friable — the high-performance GEA coater technology can process both small and large quantities of tablets at very high suspension application rates.
The operating principle of the GEA coater is based on a conventional pan and spray system, but the way the object — the tablet core — is presented to the coating spray has been improved.
The fundamental principle of the film coating process remains unchanged. Incorporating a small, simple and modular design, tablet cores (from 2.5 kg upwards) are loaded into a perforated wheel and are formed into a ring by rapidly accelerating the wheel to high speed.
Once the ring is formed, radially placed air knives induce the tablet cores into a stable, free-falling cascade, presenting the tablet cores to the coating suspension.
The drying efficiency is increased by spraying the coating suspension upwards into the cascade. As such, the process is much faster, offering a target weight gain of 3% (15% solids content) in an optimal coating time of less than 10 minutes — compared with at least 90 minutes in a conventional pan coating process.
Putting theory into practice
Tests with a flat-faced placebo core yielded promising results. Although not designed for coating and sensitive to twinning, a somewhat fragile and moisture-sensitive customer product was successfully coated without damage and only some slight twinning.
As Leslie Van Eeckhout, Senior Process Specialist at GEA, comments: “More tests would need to be done and this is not something that customers normally come to us for, but the GEA Coater can certainly be used to accomplish this task."
"The technology is well suited to handling a wide range of shapes and dimensions; it should definitely be considered as one of many GEA solutions to overcome issues with capping.”
Reference
1. M. Braun, “Scale-Up of the Tableting Process,” presentation at Granulation & Tableting — Live Online Training (13 September 2024, ECA Academy, Mannheim, Germany): www.gmp-compliance.org/training/gmp-course-conference/granulation-tableting.

