As short a time as possible between placing the order and starting production is a priority for both manufacturers and operators of pharmaceutical systems. As a supplier, Optima Pharma has created a comprehensive, systematic concept that supports this goal and leaves nothing to chance in the entire process.
The aim is to deliver new products to the market as quickly as possible. This doesn’t just apply to pharmaceuticals; but, for drug manufacturers, it is particularly important to keep the time between completing the approval phase and getting a product to market to a minimum: one, because of the lengthy development times and high development costs and, two, because international competition is increasingly keen.
The long delivery times that arise in many areas of plant engineering when capacities are stretched to their limits are a serious problem in the pharmaceutical industry.
At the same time, increased requirements are placed on the flexibility of the system. It needs to be able to handle smaller batch sizes, and various containers (such as syringes, vials and cartridges) need to be filled on one system. This makes systems increasingly complex and, normally, it would make the entire process — from design and engineering to the actual construction of the plant and validation — even lengthier.
To media fill in record time
Optima Pharma has a solution for this problem: comprehensive process engineering following a scientific approach allows the realisation of short delivery and commissioning times, together with quick qualification and a facilitated audit.
The pharmaceutical system manufacturer calls this approach comprehensive scientific process engineering … or CSPE for short. It combines tried and tested, accelerated concepts and procedures such as digital engineering, simulation, virtual reality and integrated FATs.
This minimises so-called “time thieves.” In this way, the media fill, proof that sterile products can be produced on the finished filling system, is successfully done in record time.
“We have been using these methods for quite some time,” explains Gerhard Breu, who, as Chairman of the Optima Pharma Division, is responsible for the company’s operations in Schwäbisch Hall and Mornhausen, as well as Metall+Plastic in Radolfzell, adding that the company set up its own Digital Engineering department to provide support for pharmaceutical plant construction 6 years ago.
Its repertoire includes all standard simulation methods, such as strength calculation, determination of the resonance value of individual components, etc. And, for some time now, both Optima engineers and customers have been able to experience and contribute their input on 3D system models at the VR Center in Schwäbisch Hall at an early stage of the engineering process.
Exploiting the potential of digital engineering
So what’s new about CSPE? Gerhard Breu points out the systematic approach and the consistent application of the possibilities offered by digital engineering, which, as a result of the integrated approach, becomes a necessity rather than an option. “Previously, the configuration of systems was primarily a question of experience. In-depth experience is still very much a prerequisite; but, with CSPE, we also exploit the full potential offered by digital engineering.”
The process begins even before the design stage with a project risk analysis that involves a number of specialist departments and draws parallels with similar projects. As a result, the engineers can take possible obstacles during the new project into account from the word go — and overcome them at an early stage with the help of digital engineering and simulation.
“This helps to minimise risk and facilitate problem-free commissioning,” says Breu.
He adds that, as in the past, Optima Pharma still uses real experimental set-ups in its technical centre, as this is a must with certain assemblies.
“However, our engineers aim to resolve as many issues as possible in advance using digital engineering,” the Chairman explains. For example, the distribution of VHP (vaporised hydrogen peroxide) in an isolator can be simulated.
Worst-case positions that are scarcely reached by the VHP gas are identified and the positioning of the injection nozzles in the isolator is adjusted accordingly. Simulating the sterilisation process is a great advantage for the design engineers and also supports cycle development.
The simulation shows where biological indicators would be most effective. During performance qualification (PQ), this allows the user to verify for the authorities that their system can be reliably decontaminated with hydrogen peroxide vapour.
As a further example of an application when simulation has been successfully used, Breu mentions the process of freezing vials on a freeze-dryer shelf. Following modification of the shelf design compared with a previous system, the customer wanted proof that a newly designed side guide had no negative effect.
Breu recalls: “We were able to prove that a firmly installed guide rail actually had a positive effect on the freezing process.” In this way, the customer received a welcome design modification without the need for time-consuming test constructions.
Right first time, virtually
The “high art” of digital engineering ultimately leads to the virtual mock-up, which Optima Pharma sees as an “advance virtual mock-up.”
Design engineers and future operators of a system can get an idea of the circumstances and accessibility in the VR Center, giving them a much more realistic picture than a 3D image on a computer screen. Breu doesn’t see the virtual mock-up study completely replacing the usual wooden mock-up until it becomes possible to simulate weights, tactility and haptics, too.
“With the real-life mock-up, you can test the handling of a heavy intermediate container or a sensitive component such as a filling needle using an isolator glove,” he states. However, he points out that the “first shot” in a real mock-up is significantly more accurate when bottlenecks have been eliminated in advance with the help of the virtual version. In addition, he says, the virtual system can be used for training purposes.
CSPE also works as an accelerator within the company and frees up resources: strength calculations, flow simulations or determining the resonance values of individual components support developers during the design phase.
CSPE can act as an enabler throughout the entire lifecycle of a system, acting as an aid during service call outs as part of the “Optima Total Care” portfolio, for example.
Breu remembers one specific case when the folding table on a freeze-dryer loading system had become warped. Because the customer had begun “cold loading” the mounting plate on the freeze dryer had cooled, which led to the folding table no longer being able to dock correctly.
Instead of simply sending out a service technician and hoping that they would be able to solve the problem on site by trial and error, Optima first simulated the cold-induced deformation and immediately produced a suitable component to compensate for the warping. The service technician then only needed to fit this part.
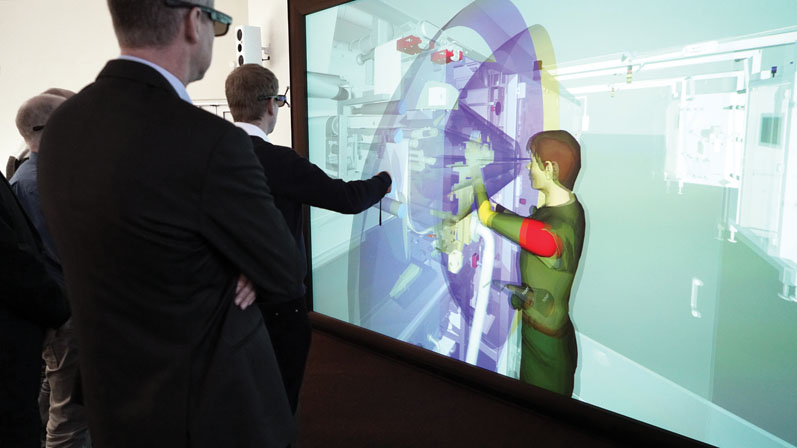
Virtual reality can’t completely replace real-life mock-ups just yet, but it can reduce a certain amount of time-consuming experimentation
Reliably qualified with integrated FAT
The integrated FAT (iFAT) is a further CSPE-related feature offered by Optima Pharma. All components of a system are brought together — such as an isolator and its freeze-dryer feed and loading system — and then tested together with the filling system under conditions that are as realistic as possible for qualification purposes (similar to the subsequent SAT).
“The only deviations that might arise later result from special features of the cleanroom environment on the customer’s premises,” says Breu. Optima is already able to do first cycle development tests and, to ensure that conditions at Optima are as realistic as possible, the company is currently building a new final assembly hall — optimally equipped for the installation and commissioning of complete lines. Construction is scheduled to be completed in the second quarter of 2019.
And with realistic system qualification as part of the iFAT, the process comes full circle. The engineering department receives direct feedback on the accuracy of the previous simulations and input to further optimise the simulation models. “Comparing your results with reality is essential if you are aiming for continual improvement,” Breu emphasises.
Turnkey + CSPE = faster time to market
System operators will see the positive effects of CSPE in many areas, such as shorter commissioning and on-site deployment times. In-depth preparatory work accelerates on-site qualification and validation via digital engineering, and iFAT means that, in some cases, it’s essentially a requalification.
This means production can get under way much faster than was previously possible. According to Breu, the amount of overall project time saved varies greatly from project to project: “It can be up to 6 months.” These effects are also heightened by the turnkey approach.
Freeze dryer, isolator and filling units supplied by one manufacturer are optimally co-ordinated as an overall system, including the qualification concept for all system components. This reduces the time to market yet again.
