A wave of counterfeit drugs is hitting the world hard … and Europe has been one of the major victims. It is estimated that 1% of medicines sold to the public in the European Union (EU) through the legal supply chain is counterfeit.
According to a report from the Pharmaceutical Security Industry (PSI), 3147 pharmaceutical crime incidents are committed a year, worldwide, with Europe reporting around 347 crimes per year.1 Furthermore, a report by the European Observatory on Infringements of Intellectual Property Rights suggests that counterfeiting costs the pharmaceutical industry and Europe more than €10 billion each year.2
This is expected to result in the loss of approximately 38,000 direct jobs. Considering the broader and indirect effects of counterfeit pharmaceuticals on other sectors, this turns out to have a negative impact of more than €17 billion, which causes 91,000 job losses and a €1.7 billion loss in government revenues.
The EU has raised concerns about counterfeit or falsified medicines and their impact on public health and safety. To counter the growing threat of counterfeiting, the EU Commission passed EU Falsified Medicines Directive (Directive 2011/62/EU) — commonly known as EU FMD.
Decoding EU FMD
The European Council and the European Parliament published the FMD on 1 July 2011. The Delegated Regulation (EU) 2016/161 to the FMD was published on 9 February 2016 and will be enforced from February 2019. The objective of the directive and the associated delegated regulation is to protect patients from falsified medicines.
The term “falsified medicines” is applied to fake medicines that are designed to mimic real medicines. The different measures mentioned in the EU FMD can be divided into three types as follows:
Safety features: As per the EU FMD, marketing authorisation holders need to introduce two safety features on the medicine’s packaging, namely a unique identifier (UI) — a two-dimensional barcode — and an antitampering device.
These safety measures must be included on the packaging of most human prescription medicines and certain non-prescription medicines on or before 9 February 2019.3
In addition, serialisation (the process of applying the UI to the packaging) must be completed at the secondary or saleable unit level. The serialised data must be then sent to a central repository for verification and government reporting.
The UI comprises four data elements (Figure 1) that must be printed in human readable form, and encoded and stored in a GS1 2D DataMatrix. The use of the fifth data element — the national reimbursement number — is optional, and few states in the EU may request to include the same in UI to link the reimbursement of a drug product under a socialised medicine programme.
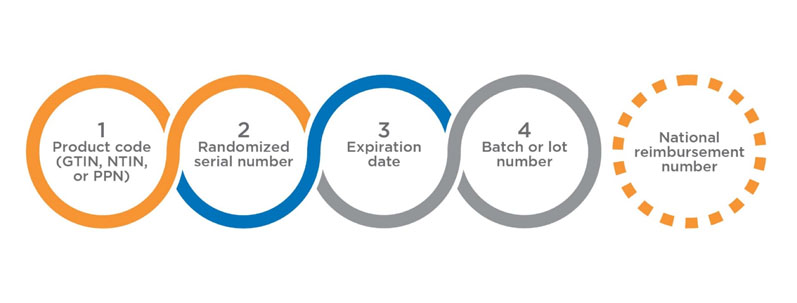
Figure 1: The UI comprises four data elements
The UI, combined with a two-dimensional barcode, can store more information than any other data carriers and serves as a robust tool with redundant information; it could still be read even if it is 80% damaged. The data encoded for EU FMD also need to be verified for correctness and quality aspects.
The second security feature that needs to be incorporated is antitampering measures for product packages. The manufacturers must apply an antitampering technology to determine whether the medicine packaging has been altered.
No specific antitampering device has been recommended by the FMD. Various measures, such as labelling (with or without perforation), hot-melt gluing with perforation, self-destroying folding boxes and wrapping can be included.
Verification of medicines at the point of dispensing: The verification of serialisation data at one or more stops along the supply chain is the next step in the EU FMD. After the manufacturers have generated the serialisation data, they must report the product master and production data to a centralised hub, known as the European Medicines Verification System (EMVS).
EMVS manages the storage of data in a database and forwards all data to the respective National Systems. The data provided by manufacturers will be validated and some of the information will be decommissioned to the National System Repositories of the target markets for the products.
When a product is dispensed through a pharmacy, it is scanned for a barcode, following which a verification enquiry for the barcode is sent to the National System supporting the member state where the pharmacy is located. The National System validates that the barcode data matches the active UI in the system. Then, the pack is decommissioned and supplied to the patient.
However, if the product fails to match the UI in the system, it will not be supplied to the patient … and the incident will be highlighted as an exceptional event, followed by an investigation to determine whether the pack has been compromised.
Online pharmacies: Internet pharmacies are one of the major sources of counterfeit medicines worldwide, mostly in remote corners of the world that are out of the reach of regulators. Online pharmacies increase the chances of criminals dodging security barriers and, therefore, have been identified and addressed by the EU FMD.
Accordingly, the directive mandates all Internet pharmacies to be authorised to supply pharmaceuticals to the public through a common logo, which will be clearly displayed on all the pages of the website and identifiable throughout the EU.
EU FMD implementation models
The EU has adopted a central repository structure (the EU hub) for data reporting and country based national repositories for verification purposes (Figure 2).
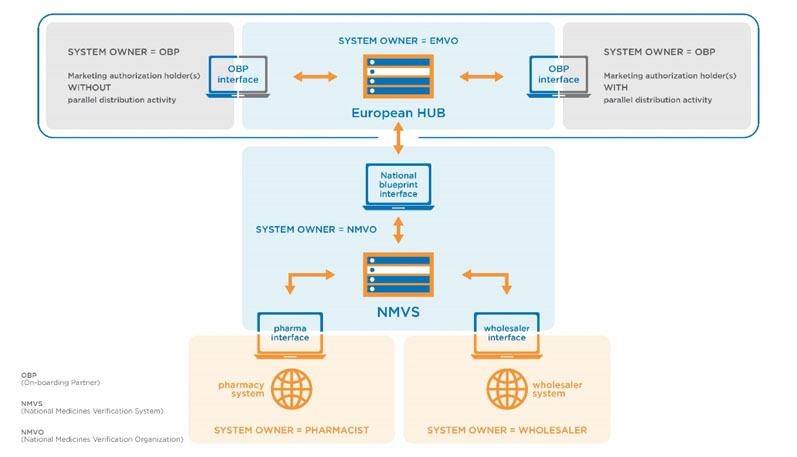
Figure 2: Central repository structure proposed by the EU as per EU FMD (https://emvo-medicines.eu/mission/emvs)
These repositories will serve the territory of one or multiple member states. Each national repository will be connected to the EU hub. Accordingly, each country of the European Economic Area and Switzerland has started implementing a National Medicines Verification System (NMVS) according to the National Medicines Verification Organization (NMVO).
NMVS shall serve as the verification platform for pharmacies and registered parties, such as wholesalers, self-dispensing doctors or hospital pharmacies for product authentication (Figure 3).4
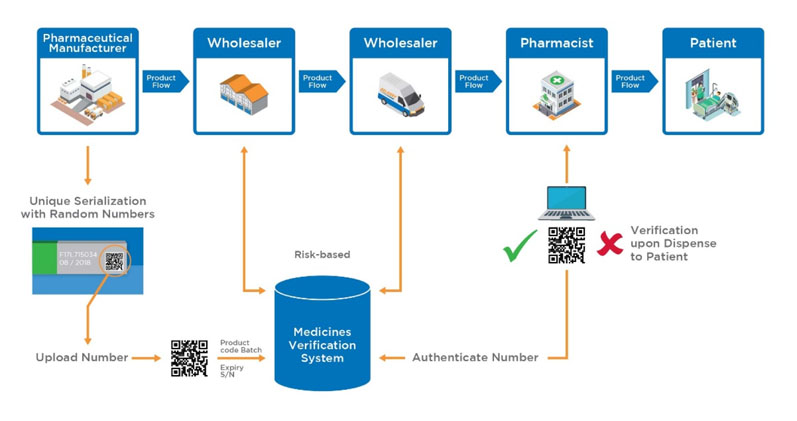
Figure 3: Proposed medicinal product flow after implementation of the EU FMD (https://emvo-medicines.eu/mission/emvs)
EU FMD: What next?
The EU FMD implementation model is expected to evolve further to incorporate complete track and trace system requirements throughout the drug supply chain. This will include aggregation at the manufacturing stage and product information tracking throughout the supply chain, including verification functionalities.
The current implementation model limits product authentication at the pharmacy level … and extension of this verification process to the consumer level is expected.
The EU FMD implementation models will serve as a base for cashless medical systems in the future. The new structure of the centralised systems will bring in complete visibility about the journey of medicines in an end-to-end pharma supply chain for government and healthcare agencies.
It will also simplify pharmaceutical transactions in EU regions, thereby providing ease of doing business to the pharmaceutical industry. Complete track and trace serialisation will safeguard pharmaceutical manufacturers from counterfeit drugs and, simultaneously, bring them closer to the customer.
References
- www.psi-inc.org/geographicDistributions.cfm.
- http://bit.ly/2QDneLZ.
- http://bit.ly/2PKZ6Wi.
- https://emvo-medicines.eu/mission/emvs.
